Abstract
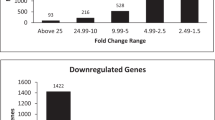
Adipose-derived stem cells (ADSCs) have been considered as the optimal cells for regenerative medicine because ADSCs have the potential of multi-directional differentiation. To study the mechanisms of ADSCs differentiation, we analyzed microarray of GSE37329. GSE37329 was downloaded from Gene Expression Omnibus including 3 ADSCs, 2 ADSCs-derived osteocytes, and 2 ADSCs-derived myocytes samples. The differentially expressed genes (DEGs) were screened using limma package. Their underlying functions were predicted by gene ontology and pathway enrichment analyses. Besides, the interaction relationships of the proteins encoded by DEGs were obtained from STRING database, and protein–protein interaction (PPI) network was constructed using Cytoscape. Furthermore, modules analysis of PPI network was performed using MCODE in Cytoscape. We screened 662 and 484 DEG separately for the ADSCs-derived osteocytes and myocytes compared with ADSCs. There were 205 common up-regulated and 128 common down-regulated DEGs between the two groups. Function enrichment indicated that these common DEGs, especially, VEGFA, FGF2, and EGR1 may be related to cell differentiation. PPI network for common DEGs also suggested that VEGFA (degree = 29), FGF2 (degree = 17), and EGR1 (degree = 12) might be more important because they had higher connectivity degrees, and they might be involved in cell differentiation by interacting with other genes in module A (e.g., EGR1–NGF and EGR1–LEP), and B (e.g., VEGFA–PDGFD). Additionally, the IGF1 and BTG1 may be, respectively, specific for osteocytes and myocytes differentiation. VEGFA, PDGFD, FGF2, EGR1, NGF, LEP, IGF1, and BTG1 might serve as target genes in regulating ADSCs differentiation.


Similar content being viewed by others
References
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Halvorsen Y-DC, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM (2001) Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism 50(4):407–413
Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F (2002) Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290(2):763–769. doi:10.1006/bbrc.2001.6270
Tian G, Zhou J, Wang Je, Xu B, Li L, Zhu F, Han J, Li J, Zhang S, Luo X (2012) Neuronal differentiation of adipose-derived stem cells and their transplantation for cerebral ischemia. Neural Regen Res 7(25):1992–1999
Kim YJ, Kim JT, Bae YC, Suh KT, Jung JS (2008) ICAT participates in proliferation and osteogenic differentiation of human adipose tissue-derived mesenchymal stem cell. Life Sci 83(25):851–858
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298
Kakudo N, Shimotsuma A, Kusumoto K (2007) Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun 359(2):239–244
Cheng N-C, Wang S, Young T-H (2012) The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 33(6):1748–1758
Cheng N-C, Chen S-Y, Li J-R, Young T-H (2013) Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Trans Med 2(8):584–594
Berdasco M, Melguizo C, Prados J, Gómez A, Alaminos M, Pujana MA, Lopez M, Setien F, Ortiz R, Zafra I (2012) DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. Am J Pathol 181(6):2079–2093
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4(2):249–264
Smyth GK (ed) (2005) Limma: linear models for microarray data. In: Bioinformatics and computational biology solutions using R and bioconductor. Springer, Berlin, pp 397–420
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 57:289–300
Hulsegge I, Kommadath A, Smits MA Globaltest and GOEAST: two different approaches for Gene Ontology analysis. In: BMC proceedings, 2009. BioMed Central Ltd, p S10
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28(1):27–30
Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, Krull M, Matys V, Michael H, Ohnhäuser R (2001) The TRANSFAC system on gene expression regulation. Nucleic Acids Res 29(1):281–283
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C (2013) STRING v9. 1: protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41(D1):D808–D815
Saito R, Smoot ME, Ono K, Ruscheinski J, Wang P-L, Lotia S, Pico AR, Bader GD, Ideker T (2012) A travel guide to cytoscape plugins. Nat Methods 9(11):1069–1076
Bader GD, Hogue CW (2003) An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinf 4(1):2
Liu DD, Zhang JC, Zhang Q, Wang SX, Yang MS (2013) TGF-β/BMP signaling pathway is involved in cerium-promoted osteogenic differentiation of mesenchymal stem cells. J Cell Biochem 114(5):1105–1114
Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH (2009) Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17(4):540–547
Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling? In control of vascular function. Nat Rev Mol Cell Biol 7(5):359–371
Street J, Bao M, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci 99(15):9656–9661
Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W (2005) Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res 20(11):2028–2035
Behr B, Tang C, Germann G, Longaker MT, Quarto N (2011) Locally applied VEGFA increases the osteogenic healing capacity of human adipose derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells (Dayton, Ohio) 29(2):286
Sotoca AM, Roelofs-Hendriks J, Boeren S, van der Kraan PM, Vervoort J, van Zoelen EJ, Piek E (2013) Comparative proteome approach demonstrates that platelet-derived growth factor C and D efficiently induce proliferation while maintaining multipotency of hMSCs. Exp Cell Res 319(17):2649–2662
Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS (2008) PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112(2):295–307
Charoenpanich A, Wall ME, Tucker CJ, Andrews DM, Lalush DS, Loboa EG (2011) Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng Part A 17(21–22):2615–2627
Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM (2000) Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest 105(8):1085–1093
Sahni M, Ambrosetti D-C, Mansukhani A, Gertner R, Levy D, Basilico C (1999) FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev 13(11):1361–1366
Hondermarck H, McLaughlin CS, Patterson SD, Bradshaw RA (1994) Early changes in protein synthesis induced by basic fibroblast growth factor, nerve growth factor, and epidermal growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci 91(20):9377–9381
Tsuboi R, Sato Y, Rifkin DB (1990) Correlation of cell migration, cell invasion, receptor number, proteinase production, and basic fibroblast growth factor levels in endothelial cells. J Cell Biol 110(2):511–517
Ito T, Sawada R, Fujiwara Y, Tsuchiya T (2008) FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-β signaling. Cytotechnology 56(1):1–7
Jin Y, Sheikh F, Detillieux KA, Cattini PA (2000) Role for early growth response-1 protein in α1-adrenergic stimulation of fibroblast growth factor-2 promoter activity in cardiac myocytes. Mol Pharmacol 57(5):984–990
Zhao H-Y, Ooyama A, Yamamoto M, Ikeda R, Haraguchi M, Tabata S, Furukawa T, Che X-F, Zhang S, Oka T (2008) Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res 68(17):7035–7041
Bajpai AK, Blaskova E, Pakala SB, Zhao T, Glasgow WC, Penn JS, Johnson DA, Rao GN (2007) 15 (S)-HETE production in human retinal microvascular endothelial cells by hypoxia: novel role for MEK1 in 15 (S)-HETE–induced angiogenesis. Invest Ophthalmol Vis Sci 48(11):4930–4938
Srivastava K, Kundumani-Sridharan V, Zhang B, Bajpai AK, Rao GN (2007) 15 (S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires STAT3-dependent expression of VEGF. Cancer Res 67(9):4328–4336
Fahmy RG, Dass CR, Sun L-Q, Chesterman CN, Khachigian LM (2003) Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med 9(8):1026–1032
Sariola H (2001) The neurotrophic factors in non-neuronal tissues. Cell Mol Life Sci 58(8):1061–1066
Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa C, Manni L, Madeddu P (2004) Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia 47(6):1047–1054
Friedman WJ, Greene LA (1999) Neurotrophin signaling via Trks and p75. Exp Cell Res 253(1):131–142. doi:10.1006/excr.1999.4705
Pilgaard L, Lund P, Duroux M, Lockstone H, Taylor J, Emmersen J, Fink T, Ragoussis J, Zachar V (2009) Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res 315(11):1937–1952
Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A (2005) Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 37(5):607–621
Weiss S, Baumgart R, Jochum M, Strasburger C, Bidlingmaier M (2002) Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res 17(7):1280–1289
Tang KT, Capparelli C, Stein JL, Stein GS, Lian JB, Huber AC, Braverman LE, DeVito WJ (1996) Acidic fibroblast growth factor inhibits osteoblast differentiation in vitro: altered expression of collagenase, cell growth-related, and mineralization-associated genes. J Cell Biochem 61(1):152–166
Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA (1999) Growth factor regulation of fracture repair. J Bone Miner Res 14(11):1805–1815
Eingartner C, Coerper S, Fritz J, Gaissmaier C, Koveker G, Weise K (1999) Growth factors in distraction osteogenesis. Int Orthop 23(5):253–259
Canalis E, McCarthy TL, Centrella M (1991) Growth factors and cytokines in bone cell metabolism. Annu Rev Med 42(1):17–24
Wang S, Mu J, Fan Z, Yu Y, Yan M, Lei G, Tang C, Wang Z, Zheng Y, Yu J (2012) Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res 8(3):346–356
Bogdan J, Adams-Burton C, Pedicord D, Sukovich D, Benfield P, Corjay M, Stoltenborg J, Dicker I (1998) Human carbon catabolite repressor protein (CCR4)-associative factor 1: cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1. Biochem J 336:471–481
Busson M, Carazo A, Seyer P, Grandemange S, Casas F, Pessemesse L, Rouault J-P, Wrutniak-Cabello C, Cabello G (2005) Coactivation of nuclear receptors and myogenic factors induces the major BTG1 influence on muscle differentiation. Oncogene 24(10):1698–1710
Kamaid A, Giráldez F (2008) Btg1 and Btg2 gene expression during early chick development. Dev Dyn 237(8):2158–2169
Acknowledgments
This study was supported by The Inner Mongolia autonomous region natural funded projects (No. 2013MS1194) and National Natural Science Foundation of China (No. 31260230).
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yizhong Ren and Changxu Han have contributed equally to this work.
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
About this article
Cite this article
Ren, Y., Han, C., Wang, J. et al. RETRACTED ARTICLE: Identification of genes associated with the differentiation potential of adipose-derived stem cells to osteocytes or myocytes. Mol Cell Biochem 400, 135–144 (2015). https://doi.org/10.1007/s11010-014-2269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2269-y