Abstract
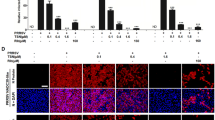
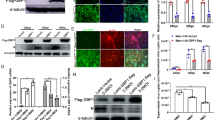
Porcine reproductive and respiratory syndrome virus (PRRSV) is considered as a significant contributor to porcine reproductive and respiratory syndrome, one of the most economically important diseases for the pig industry worldwide. Emerging evidence indicates that pattern recognition receptors play key roles in recognizing pathogen-associated molecular patterns. In the present study, we investigated the effects of a novel pattern recognition receptor LSM14A in regulating PRRSV replication. Results in Marc-145 cells and porcine alveolar macrophages (PAMs) indicated that overexpression of porcine LSM14A effectively inhibited the replication of PRRSV, and knockdown of LSM14A by siRNA enhanced the replication of PRRSV. Mechanistically, LSM14A up-regulated the activities of IFN-β and ISRE promoters, enhanced the production of IFN-β, RIG-I, and ISGs, and inhibited the production of the inflammatory cytokines of TNF-α and IL-6 mRNA. Additionally, the expression pattern of LSM14A during the infection of PRRSV in Tongcheng and Large White pigs was suppressed by the PRRSV challenge. Taken together, our results suggest that LSM14A is an important PRR that inhibits PPRSV replication by inducing IFN-β signaling and restraining inflammatory responses. Furthermore, the down-regulation of LSM14A by PRRSV might represent an important mechanism by which PRRSV invades the host. Our study sheds light on the possibility of developing a new strategy to control this disease.






Similar content being viewed by others
References
Snijder EJ, Meulenberg JJM (1998) The molecular biology of arteriviruses. J Gen Virol 79:961–979
Allende R, Lewis TL, Lu Z, Rock DL, Kutish GF, Ali A, Doster AR, Osorio FA (1999) North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J Gen Virol 80:307–315
Meulenberg JJ (2000) PRRSV, the virus. Vet Res 31:11–21
Rossow KD (1998) Porcine reproductive and respiratory syndrome. Vet Pathol 35:1–20
Holtkamp D, Kliebenstein J (2011) PRRS Costs industry $664 million annually. Pork Checkoff Study [http://www.pork.org/News/1265/PRRSCostsIndustry 664 Million.aspx]
Chang CC, Yoon KJ, Zimmerman JJ, Harmon KM, Dixon PM, Dvorak CM, Murtaugh MP (2002) Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J Virol 76:4750–4763
Drew TW (2000) A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Vet Res 31:27–39
Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988
Miller LC, Laegreid WW, Bono JL, Chitko-McKown CG, Fox JM (2004) Interferon type I in porcine reproductive and respiratory syndrome virus-infected Marc-145 cells. Arch Virol 149:2453–2463
Murtaugh MP, Xiao Z, Zuckermann F (2002) Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol 15:533–547
Luo R, Xiao S, Jiang Y, Jin H, Wang D, Liu M, Chen H, Fang L (2008) Porcine reproductive and respiratory syndrome virus (PRRSV) suppresses interferon-beta production by interfering with the RIG-I signaling pathway. Mol Immunol 45:2839–2846
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Pichlmair A, Reise Sousa C (2007) Innate recognition of viruses. Immunity 27:370–383
Barbalat R, Ewald SE, Mouchess ML, Barton GM (2011) Nucleic acid recognition by the innate immune system. Annu Rev Immunol 29:185–214
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650
Unterholzner L, Keating SE, Baranl M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowiel AG (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004
Zhang ZQ, Yuan B, Bao MS, Lu N, Kim T, Liu YJ (2011) The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12:959–965
Li Y, Chen R, Zhou Q, Xu Z, Li C, Wang S, Mao A, Zhang X, He W, Shu HB (2012) LSm14A is a processing body-associated sensor of viral nucleic acids that initiates cellular antiviral response in the early phase of viral infection. Proc Natl Acad Sci 109:11770–11775
Li B, Xiao S, Wang Y, Xu S, Jiang Y, Chen H, Fang L (2009) Immunogenicity of the highly pathogenic porcine reproductive and respiratory syndrome virus GP5 protein encoded by a synthetic ORF5 gene. Vaccine 27:1957–1963
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25:402–408
Hosseini SY, Sabahi F, Moazzeni SM, Modarressi MH, Saberi Firoozi M, Ravanshad M (2012) Construction and preparation of three recombinant adenoviruses expressing truncated NS3 and core genes of hepatitis C virus for vaccine purposes. Hepatitis monthly 12:e6130
Zhou P, Zhai SL, Zhou X, Lin P, Jiang TF, Hu XY, Jiang YB, Wu B, Zhang QD, Xu XW, Li JP, Liu B (2011) Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo. Int J Biol Sci 7:947–958
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reise Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449:819–826
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T (2004) The RNA helicase RIG-I has an essential function in double stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737
Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ (2006) The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24:633–642
Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7:131–137
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420:1–16
Takeuchi O, Akira S (2009) Innate immunity to virus infection. Immunol Rev 227:75–86
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Taniguchi T, Takaoka A (2001) A weak signal for strong responses: interferon- alpha/beta revisited. Nat Rev Mol Cell Biol 2:378–386
Wang C, Chen TY, Zhang J, Yang MJ, Li N, Xu XF, Cao XT (2009) The E3 ubiquitin ligase Nrdp1 ‘preferentially’ promotes TLR-mediated production of type I interferon. Nat Immunol 10:744–753
Dai J, Pan W, Wang P (2011) ISG15 facilitates cellular antiviral response to dengue and west Nile virus infection in vitro. Virol J 8:468
Zhang D, Zhang DE (2011) Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res 31:119–130
Liu C, Li X, Yao X, Kong XH, Qiao WT, Geng YQ (2010) Short report Bovine ISG15: an antiviral and inducible protein in BIV infected fetal bovine lung cells. Virol J 7:134
Haller O, Kochs G, Human MxA protein (2011) An interferon-induced dynamin- like GTPase with broad antiviral activity. J Interferon Cytokine Res 31:79–87
Patzina C, Haller O, Kochs G (2014) Structural requirements for the antiviral activity of the human MxA protein against thogoto and influenza a virus. J Biol Chem 289:6020–6027
Xiao H, Killip MJ, Staeheli P, Randall RE, Jackson D (2013) The human interferon-induced MxA protein inhibits early stages of influenza a virus infection by retaining the incoming viral genome in the cytoplasm. J Virol 87:13053–13508
Zhang L, Liu J, Bai J, Du Y, Wang X, Liu X, Jiang P (2013) Poly(I:C) inhibits porcine reproductive and respiratory syndrome virus replication in MARC-145 cells via activation of IFIT3. Antiviral Res 99:197–206
Liew FY, Xu D, Brint EK, O’Neill LA (2005) Negative regulation of toll-like receptor mediated immune responses. Nat Rev Immunol 5:446–458
Acknowledgments
This study was supported by the Major International Cooperation NSFC (31210103917) and the National High Technology Research and Development Program of China (2011AA100304). We thank Professor Shaobo Xiao (Huazhong Agriculture University, Wuhan, China) for generously providing the PRRSV strain WUH3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Chen, R., Zhao, J. et al. LSM14A inhibits porcine reproductive and respiratory syndrome virus (PRRSV) replication by activating IFN-β signaling pathway in Marc-145. Mol Cell Biochem 399, 247–256 (2015). https://doi.org/10.1007/s11010-014-2251-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2251-8