Abstract
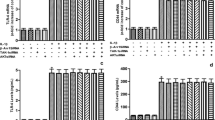
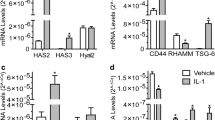
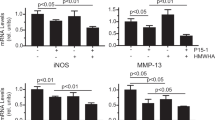
Beta-arrestin-2 is an adaptor protein that terminates G protein activation and seems to be involved in the modulation of the inflammatory response. Small hyaluronan (HA) fragments, such as 4-mer HA oligosaccharides, are known to interact with the toll-like receptor-4 (TLR-4) with consequent activation of the nuclear factor kappaB (NF-kB) that in turn stimulates the inflammation response. NF-kB activation is mediated by different pathways, in particular by the transforming growth factor-activated kinase-1 (TAK-1). Conversely, increased levels of protein kinase A (PKA), induced by cyclic adenosine monophosphate (cAMP), seem to inhibit NF-kB activation. We studied the involvement and role of beta-arrestin-2 in mouse chondrocytes stimulated with 4-mer HA fragments. The exposure of chondrocytes to 4-mer HA produced a significant up-regulation in TLR-4, cAMP, beta-arrestin-2, TAK-1, protein 38 mitogen-activated protein kinase (p38MAPK), and PKA, both in terms of mRNA expression and of the related protein levels. NF-kB was significantly activated, thereby producing the transcription of pro-inflammatory mediators, including tumor necrosis factor alpha, interleukin-6, and interleukin-17. The treatment of 4-mer HA-stimulated chondrocytes with antibodies against beta-arrestin-2 and/or a specific PKA inhibitor, significantly increased the inflammatory response, while the treatment with a specific p38MAPK inhibitor significantly reduced the inflammatory response. Interestingly, the anti-inflammatory action exerted by beta-arrestin-2 appeared to be mediated in part through the direct inhibition of p38MAPK, preventing NF-kB activation, and in part through cAMP and PKA activation primed by G protein signaling, which exerted an inhibitory effect on NF-kB. Taken together, these results could be useful for future anti-inflammatory strategies.




Similar content being viewed by others
References
Tian X, Kang DS, Benovic JL (2014) β-arrestins and G protein-coupled receptor trafficking. Handb Exp Pharmacol 219:173–186
Violin JD, Lefkowitz RJ (2007) β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422
DeFea KA (2011) Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal 23:621–629
Fan H, Bitto A, Zingarelli B, Luttrell LM, Borg K, Halushka PV, Cook JA (2010) Beta-arrestin-2 negatively regulates sepsis-induced inflammation. Immunology 130:344–351
Fan H (2013) β-arrestins 1 and 2 are critical regulators of inflammation. Innate Immun 20:451–460
Jiang D, Xie T, Liang J, Noble PW (2013) Beta-Arrestins in the immune system. Prog Mol Biol Transl Sci 118:359–393
Li P, Cook JA, Gilkeson GS, Luttrell LM, Wang L, Borg KT, Halushka PV, Fan H (2011) Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol 49:64–74
Ospelt C, Gay S (2008) The role of resident synovial cells in destructive arthritis. Best Pract Res Clin Rheumatol 22:239–252
Maquart FX, Bellon G, Pasco S, Monboisse JC (2005) Matrikines in the regulation of extracellular matrix degradation. Biochimie 87:353–360
Adair-Kirk TL, Senior SM (2008) Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 40:1101–1110
Jiang D, Liang J, Noble PW (2011) Hyaluronan as an immune regulator in human diseases. Physiol Rev 91:221–264
Jiang D, Liang J, Noble PW (2007) Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23:435–461
Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 165:1863–1870
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Calatroni A (2009) Differential effect of molecular size HA in mouse chondrocytes stimulated with PMA. Biochim Biophys Acta 1790:1353–1367
Li Z, Potts EN, Piantadosi CA, Foster WM, Hollingsworth JW (2010) Hyaluronan fragments contribute to the ozone-primed immune response to lipopolysaccharide. J Immunol 185:6891–6898
Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A (2010) Small hyaluronan oligosaccharides induce inflammation by both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol 80:480–490
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Calatroni A, Campo S (2012) 6-Mer hyaluronan oligosaccharides increase IL-18 and IL-33 production in mouse synovial fibroblasts subjected to collagen-induced arthritis. Innate Immun 18:675–684
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S (2012) Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. BioFactors 38:69–76
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S (2012) The stimulation of adenosine A2 receptor reduces inflammatory response in mouse articular chondrocytes treated with hyaluronan oligosaccharides. Matrix Biol 31:338–351
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S (2013) 4-Mer hyaluronan oligosaccharides stimulate inflammation response in synovial fibroblasts in part via TAK-1 and in part via P38-MAPK. Curr Med Chem 20:1162–1172
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Nastasi G, Micali A, Puzzolo D, Pisani A, Calatroni A, Campo S (2013) The SOD mimic MnTM-2-PyP(5 +) reduces hyaluronan degradation-induced inflammation in mouse articular chondrocytes stimulated with Fe(II) plus ascorbate. Int J Biochem Cell Biol 45:1610–1619
Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on toll-like receptor 4, CD44, and MD-2. J Biol Chem 282:18265–18275
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11:1173–1179
Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A (2010) Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie 92:204–215
Campo GM, Avenoso A, D’Ascola A, Nastasi G, Micali A, Puzzolo D, Pisani A, Prestipino V, Scuruchi M, Calatroni A, Campo S (2013) Combined treatment with hyaluronan inhibitor Pep-1 and a selective adenosine A2 receptor agonist reduces inflammation in experimental arthritis. Innate Immun 19:462–478
Sun JS, Wu CX, Tsuang YH, Chen LT, Sheu SY (2006) The in vitro effects of dehydroepiandrosterone on chondrocyte metabolism. Osteoarthr Cartil 14:238–249
Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwatz DA, Lefkowitz RJ (2003) Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112:566–574
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G (2004) Identification of β-arrestin2 as a G-protein-coupled receptor-stimulated regulator of NF-kB pathways. Mol Cell 14:303–317
Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ (2004) β-Arrestin inhibits NF-kB activation by means of its interaction with the NF-kB inhibitor IkBα. Proc Natl Acad Sci USA 101:8603–8607
Sun Y, Cheng Z, Ma L, Pei G (2002) β-arrestin-2 is critically involved in CXCR4-mediated chemotaxis and this is mediated by its enhancement of p38MAPK activation. J Biol Chem 277:49212–49219
Noss EH, Brenner MB (2008) The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev 223:252–270
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, Calatroni A, Campo S (2012) Adenosine A2A receptor activation and hyaluronan fragment inhibition reduce inflammation in mouse articular chondrocytes stimulated with interleukin-1β. FEBS J 279:2120–2133
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, Calatroni A, Campo S (2012) The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem 113:1852–1867
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S (2012) Inhibition of hyaluronan synthesis reduced inflammatory response in mouse synovial fibroblasts subjected to collagen-induced arthritis. Arch Biochem Biophys 518:42–52
Acknowledgments
This study was supported by a Grant COFIN 2009 from the MIUR, Italy [Grant n° 20094C2H2M_003].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campo, G.M., Avenoso, A., D’Ascola, A. et al. Beta-arrestin-2 negatively modulates inflammation response in mouse chondrocytes induced by 4-mer hyaluronan oligosaccharide. Mol Cell Biochem 399, 201–208 (2015). https://doi.org/10.1007/s11010-014-2246-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2246-5