Abstract
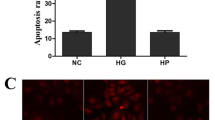
Previously, we confirmed that taurine prevented diabetes-induced apoptosis in retinal glial cells via its anti-oxidation and anti-glutamate excitotoxicity mechanisms. The aim of this study is to investigate the effects of taurine on angiopoietin-2 (Ang-2)/Tie-2 system expressions and apoptosis in high glucose-treated retinal microvascular pericytes (RMPs). Also, the possible mechanism involved in the inhibition of taurine on RMPs apoptosis is investigated. The expressions of Ang-2, Tie-2 were detected by qRT-PCR and ELISA. The level of phosphorylated Tie-2 (P-Tie-2) was examined by ELISA. Hoechst 33342 and Annexin V/PI staining were used to detect RMPs apoptosis. The activity of caspase-3 was detected by assay kit. In 25 mM high glucose group, the expression of Ang-2 was increased significantly, taurine down-regulated Ang-2 in a dose (0.1, 1, and 10 mM)-dependent manner (P < 0.05). The Tie-2 expression and P-Tie-2 level were decreased in high glucose group (P < 0.05). Interestingly, taurine at 1 and 10 mM showed significant increase in Tie-2 expression and P-Tie-2 level (P < 0.05). The number of apoptotic RMPs and the activity of caspase-3 increased in the presence of high glucose (P < 0.05). Treatment with taurine at 1 mM decreased the number of apoptotic RMPs and the activity of caspase-3 (P < 0.05). Blocking antibody and small interfering RNA (siRNA) treatment showed that taurine required Tie-2 to perform its anti-apoptotic effect. Taken together, our data suggest that high glucose-induced Ang-2/Tie-2 system expressions alteration can be reversed by taurine, and that taurine can inhibit high glucose-induced RMPs apoptosis via Tie-2.






Similar content being viewed by others
References
Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE (2008) The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 115:1859–1868
Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S (2006) Diabetic retinopathy in a multi-ethniccohort in the United States. Am J Ophthalmol 141:446–455
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Aiello LM (2003) Perspectives on diabetic retinopathy. Am J Ophthalmol 136:122–135
Klein BE, Klein R, Moss SE, Palta M (1995) A cohort study of the relationship of diabetic retinopathy to blood pressure. Arch Ophthalmol 113:601–606
Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP (2008) Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57:2495–2502
Kim J (2004) Pericytes and the prevention of diabetic retinopathy. Diabetes Res Clin Pract 66:49–51
Kutcher ME, Kolyada AY, Surks HK, Herman IM (2007) Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol 171:693–701
Zou MH, Li H, He C, Lin M, Lyons TJ, Xie Z (2011) Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosisi-n diabetes. Am J Pathol 179:2835–2844
Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME (2008) The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopa-thy. Invest Ophthalmol Vis Sci 49:2163–2171
Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y (2003) Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci 4:393–402
Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ (1999) Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem 274:30896–30905
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Puri MC, Bernstein A (2003) Requirement for the TIE family of receptortyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA 100:12753–12758
Park YS, Kim NH, Jo I (2003) Hypoxia and vascular endothelial growthfactor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovineretinal pericytes. Microvasc Res 65:125–131
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, aligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180
Hammes HP, Lin J, Wagner P, Feng Y, vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H (2005) Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 139:476–481
Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y (2010) Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol 47:59–64
Feng Y, vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, Wagner P, Lin J, Deutsch U, Hammes HP (2007) Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost 97:99–108
Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A (2011) A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci 52:3784–3791
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY (2001) Role of taurine in regulation of intracellular calcium level and neuroprotective function incult-ured neurons. J Neurosci Res 66:612–619
Di Leo MA, Santini SA, Cercone S, Lepore D, Silveri NG, Caputo S, Greco AV, Giardina B, Franconi F, Ghirlanda G (2002) Chronic taurine supplementation ameliorates oxidative stress and Na + K + ATPase impairment in the retina of diabetic rats. Amino Acids 23:401–406
Kaplan B, Karabay G, Zağyapan RD, Ozer C, Sayan H, Duyar I (2004) Effects of taurine in glucose and taurine administration. Amino Acids 27:327–333
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17:330–346
Franconi F, Di Leo MA, Bennardini F, Ghirlanda G (2004) Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 29:143–150
Franconi F, Loizzo A, Ghirlanda G, Seghieri G (2006) Taurine supplementation and diabetes mellitus. Curr Opin Clin Nutr Metab Care 9:32–36
Di Leo MA, Ghirlanda G, Silveri NG, Giardina B, Franconi F, Santini SA (2003) Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic Res 37:323–330
De Luca G, Calpona PR, Caponetti A, Romano G, Di Benedetto A, Cucinotta D, Di Giorgio RM (2001) Taurine and osmoregulation: platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 50:60–64
Yu X, Xu Z, Mi M, Xu H, Zhu J, Wei N, Chen K, Zhang Q, Zeng K, Wang J, Chen F, Tang Y (2008) Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of gl-utamate in streptozotocin-induced Sprague-Dawley rats. Neurochem Res 33:500–507
Zeng K, Xu H, Mi M, Zhang Q, Zhang Y, Chen K, Chen F, Zhu J, Yu X (2009) Dietary taurine supplementation prevents glial alterations in retina of diabetic rats. Neurochem Res 34:244–254
Zeng K, Xu H, Chen K, Zhu J, Zhou Y, Zhang Q, Mantian M (2010) Effects of taurine on glutamate uptake and degradation in Müller cells under diabetic conditions via antioxidant mechanism. Mol Cell Neurosci 45:192–199
Zeng K, Xu H, Mi M, Chen K, Zhu J, Yi L, Zhang T, Zhang Q, Yu X (2010) Effects of taurine on glial cells apoptosis and taurine transporter expression in retina under di-abetic conditions. Neurochem Res 35:1566–1574
Ueki I, Stipanuk MH (2007) Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 137:1887–1894
Hayes KC, Rabin AR, Berson EL (1975) An ultrastructural study of nutritionally induced and reversed retinal degeneration in cats. Am J Pathol 78:505–524
Juen S, Kieselbach GF (1990) Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol 108:372–375
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM (1998) Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy Penn State Retina Research Group. Diabetes 47:815–820
Barber AJ, Antonetti DA, Gardner TW (2000) Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabet-es. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci 41:3561–3568
Bernardi N (1985) On the role of taurine in the cerebellar cortex: a reappraisal. Acta Physiol Pharmacol Latinoam 35:153–164
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res 29:189–197
Thurston JH, Hauhart RE, Dirgo JA (1980) Taurine: a role in osmotic regulation of mammalian brain and possible clinical significance. Life Sci 26:1561–1568
Ohashi H, Takagi H, Koyama S, Oh H, Watanabe D, Antonetti DA, Matsubara T, Nagai K, Arai H, Kita T, Honda Y (2004) Alterations in expression of angiopoietins and the Tie-2 receptor in the retina of streptozotocin induced diabetic rats. Mol Vis 10:608–617
Alikhani M, Roy S, Graves DT (2010) FOXO1 plays an essential role in apoptosis of retinal pericytes. Mol Vis 16:408–415
Haribalaganesh R, Sheikpranbabu S, Elayappan B, Venkataraman D, Gurunatha S (2009) Pigment epithelium-derived factor down regulates hyperglycemia-induced apoptosis via PI3 K/Akt activationin goat retinal pericytes. Angiogenesis 2:381–389
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant 81202206). This work is also supported by the Public-Health-Department-Foundation of Sichuan, China (Grant 100533).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaihong Zeng, Jian Ming: These authors contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Zeng, K., Ming, J., Yang, N. et al. Taurine prevents high glucose-induced angiopoietin-2/tie-2 system alterations and apoptosis in retinal microvascular pericytes. Mol Cell Biochem 396, 239–248 (2014). https://doi.org/10.1007/s11010-014-2159-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2159-3