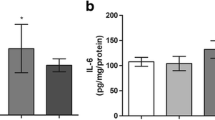
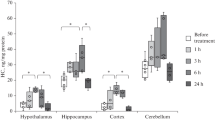
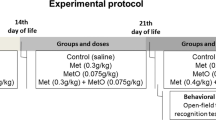
Abstract
Methionine is the only endogenous precursor of homocysteine, sulfur—containing amino acid and well known as risk factor for various brain disorders. Acetylcholinesterase is a serine protease that rapidly hydrolyzes neurotransmitter acetylcholine. It is widely distributed in different brain regions. The aim of this study was to elucidate the effects of methionine nutritional overload on acetylcholinesterase activity in the rat brain. Males of Wistar rats were randomly divided into control and experimental group, fed from 30th to 60th postnatal day with standard or methionine-enriched diet (double content comparing to standard, 7.7 g/kg), respectively. On the 61st postnatal day, total homocysteine concentration was determined and showed that animals fed with methionine-enriched diet had significantly higher serum total homocysteine concentrations comparing to control rats (p < 0.01). Acetylcholinesterase activity has been determined spectrophotometrically in homogenates of the cerebral cortex, hippocampus, thalamus, and nc. caudatus. Acetylcholinesterase activity showed tendency to decrease in all examined brain structures in experimental comparing to control rats, while statistical significance of this reduction was achieved in the cerebral cortex (p < 0.05). Brain slices were stained with haematoxylin and eosin (H&E) and observed under light microscopy. Histological analysis of H&E-stained brain slices showed that there were no changes in the brain tissue of rats which were on methionine-enriched diet compared to control rats. Results of this study showed selective vulnerability of different brain regions on reduction of acetylcholinesterase activity induced by methionine-enriched diet and consecutive hyperhomocysteinemia.



Similar content being viewed by others
References
Finkelstein JD (1998) The metabolism of homocysteine: pathways and regulation. Eur J Pediatr 157(Suppl. 2):S40–S44
Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19:217–246. doi:10.1146/annurev.nutr.19.1.217
Hoffer LJ (2004) Homocysteine remethylation and trans-sulfuration. Metabolism 53(11):1480–1483. doi:10.1016/j.metabol.2004.06.003
McCully KS (1969) Vascular pathology of homocysteinemia: implications for thepathogenesis of arteriosclerosis. Am J Pathol 56(1):111–112
Djurić D, Jakovljević V, Rašić-Marković A, Đurić A, Stanojlović O (2008) Homocysteine, folic acid and coronary artery disease: possible impact on prognosis and therapy. Indian J Chest Di Allied Sci 50:39–48
Herrmann W, Obeid R (2011) Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med 49(3):435–441. doi:10.1515/CCLM.2011.084
Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483. doi:10.1056/NEJMoa011613
Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP (2002) Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem 80:101–110. doi:10.1046/j.0022-3042.2001.00676.x
Sachdev P (2004) Homocysteine, cerebrovascular disease and brain atrophy. J Neurol Sci 226:25–29. doi:10.1016/j.jns.2004.09.006
Stanojlović O, Rašić-Marković A, Hrnčić D, Šušić V, Macut Dj, Radosavljević T, Djurić D (2009) Two types of seizures in homocysteine thiolactone–treated adult rats, behavioral and encephalographic study. Cell Mol Neurobiol 29:329–339. doi:10.1007/s10571-008-9324-8
Jakubowski H (2004) Molecular basis of homocysteine toxicity in humans. Cell Mol Life Sci 61:470–487. doi:10.1007/s00018-003-3204-7
Perla-Kajan J, Twardowski T, Jakubowski H (2007) Mechanisms of homocysteine toxicity in humans. Amino Acids 32:561–572
Troen AM (2005) The central nervous system in animal models of hyperhomocysteinemia. Prog Neuropsychopharmacol Biol Psychiatry 29:1140–1151. doi:10.1016/j.pnpbp.2005.06.025
Hrnčić D, Rašić-Marković A, Krstić D, Macut D, Djuric D, Stanojlović O (2010) The role of nitric oxide in homocysteine thiolactone-induced seizures in adult rats. Cell Mol Neurobiol 30:219–231. doi:10.1007/s10571-009-9444-9
Rasić-Marković A, Stanojlović O, Hrnčić D, Krstić D, Čolović M, Šusić V, Radosavljević T, Djuric D (2009) The activity of erythrocyte and brain Na+/K+ and Mg2+-ATPases in rats subjected to acute homocysteine and homocysteine thiolactone administration. Mol Cell Biochem 327:39–45
Prado MA, Reis RA, Prado VF, de Mello MC, Gomez MV, de Mello FG (2002) Regulation of acetylcholine synthesis and storage. Neurochem Int 41(5):291–299. doi:10.1016/S0197-0186(02)00044-X
Gralewicz S (2006) Possible consequences of acetylcholinesterase inhibition in organophosphate poisoning. Discussion continued. Med Pr 57(3):291–302
Schulpis K, Kalimeris K, Bakogiannis C, Tsakiris T, Tsakiris S (2006) The effect of in vitro homocystinuria on the suckling rat hippocampal acetylcholinesterase. Metab Brain Dis 21(1):21–28. doi:10.1007/s11011-006-9001-x
Stefanello FM, Zugno AI, Wannmacher CM, Wajner M, Wyse AT (2003) Homocysteine inhibits butyrylcholinesterase activity in rat serum. Metab Brain Dis 18(3):187–194. doi:10.1023/B:MEBR.0000020189.89585.3b
Scherer EB, da Cunha AA, Kolling J, da Cunha MJ, Schmitz F, Sitta A, Lima DD, Delwing D, Vargas CR, Wyse AT (2011) Development of an animal model for chronic mild hyperhomocysteinemia and its response to oxidative damage. Int J Dev Neurosci 29(7):693–699. doi:10.1016/j.ijdevneu.2011.06.004
Petrović M, Fufanović I, Elezović I, Čolović M, Krstić D, Jakovljević V, Đurić D (2010) The effect of homocysteine thiolactone on acetylcholinesterase activity in rat brain, blood and brain. Ser J Exp Clin Res 11(1):19–22
Darvesh S, Walsh R, Martin E (2007) Homocysteine thiolactone and human cholinesterases. Cell Mol Neurobiol 27(1):33–48. doi:10.1007/s10571-006-9114-0
Whittaker VP, Barker LA (1972) The subcellular fractionation of brain tissue with special reference to the preparation of synaptosomes and their component organelles. Method Neurochem 2:1–52
Ellman GL, Courtney KD, Anres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
De Bree A, Verschuren WM, Kromhout D, Kluijtmans LA, Blom HJ (2002) Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev 54(4):599–618. doi:10.1124/pr.54.4.599
Pexa A, Herrmann M, Taban-Shomal O, Henle T, Deussen A (2009) Experimental hyperhomocysteinaemia: differences in tissue metabolites between homocystine and methionine feeding in a rat model. Acta Physiol (Oxf) 197(1):27–34. doi:10.1111/j.1748-1716.2009.01981.x
Dayal S, Arning E, Bottiglieri T, Böger RH, Sigmund CD, Faraci FM, Lentz SR (2004) Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 35(8):1957–1962
Blaise SA, Nédélec E, Schroeder H, Alberto JM, Bossenmeyer-Pourié C, Guéant JL, Daval JL (2007) Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am J Pathol 170(2):667–679
Fukada S, Shimada Y, Morita T, Sugiyama K (2006) Suppression of methionine-induced hyperhomocysteinemia by glycine and serine in rats. Biosci Biotechnol Biochem 70(10):2403–2409
Matté C, Mackedanz V, Stefanello FM, Scherer EB, Andreazza AC, Zanotto C, Moro AM, Garcia SC, Gonçalves CA, Erdtmann B, Salvador M, Wyse AT (2009) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood of rats: protective effect of folic acid. Neurochem Int 54(1):7–13
Schweinberger BM, Schwieder L, Scherer E, Sitta A, Vargas CR, Wyse AT (2014) Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+, K+-ATPase/Mg2+-ATPase activity and oxidative status of the offspring. Metab Brain Dis 29(1):153–160. doi:10.1007/s11011-013-9451-x
Kolling J, Scherer EB, Siebert C, Marques EP, Dos Santos TM, Wyse AT (2014) Creatine prevents the imbalance of redox homeostasis caused by homocysteine in skeletal muscle of rats. Gene 545(1):72–79. doi:10.1016/j.gene.2014.05.005
Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudy PV, Arnelle DR, Stamler JS (1997) Neurotoxcity associated with dual actions of Homocysteine at the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 94:5923–5928. doi:10.1073/pnas.94.11.5923
Lazarewicz JW, Ziembowicz A, Matyja E, Stafiej A, Zieminska E (2003) Homocysteine-evoked 45Ca release in the rabbit hippocampus is mediated by both NMDA and group I metabotropic glutamate receptors: in vivo microdialysis study. Neurochem Res 28:259–269
Zou CG, Zhao YS, Gao SY, Li SD, Cao XZ, Zhang M, Zhang KQ (2010) Homocysteine promotes proliferation and activation of microglia. Neurobiol Aging 31(12):2069–2079. doi:10.1016/j.neurobiolaging.2008.11.007
Shi QS, Savage JE, Hufeisen SJ, Rauser L, Grajkowska E, Ernsberger P (2003) l-Homocysteine sulfinic acid and other acidic homocysteine derivates are potent and selective metabotropic glutamate receptor agonists. J Pharmacol Exp Ther 305:131–142. doi:10.1124/jpet.102.047092
Hrnčić D, Rašić-Marković A, Krstić D, Dj Macut, Šušić V, Djuric D, Stanojlović O (2012) Inhibition of the neuronal nitric oxide synthase potentiates homocysteine thiolactone—induced seizures in adult rats. Med Chem 8(1):59–64. doi:10.2174/157340612799278577
Ramakrishnan S, Sulochana KN, Lakshmi S, Selvi R, Angayarkanni N (2006) Biochemistry of homocysteine in health and diseases. Indian J Biochem Biophys 43:275–283
Zhou JF, Zhou W, Zhang SM, Luo YE, Chen HH (2004) Oxidative stress and free radical damage in patients with acute dipterex poisoning. Biomed Environ Sci 17:223–233
Tsakiris S, Angelogianni P, Schulpis KH, Stavridis JC (2000) Protective effect of l-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin Biochem 33(2):103–106. doi:10.1016/S0009-9120(99)00090-9
Vučević D, Petronijević N, Radonjić N, Rašić-Marković A, Mladenović D, Radosavljević T, Hrnčić D, Djuric D, Sušić V, Dj Macut, Stanojlović O (2009) Acetylcholinesterase as a potential target of acute neurotoxic effects of lindane in rats. Gen Physiol Biophys 8:18–24
Mikroulis AV, Psarropoulou C (2012) Endogenous ACh effects on NMDA-induced interictal-like discharges along the septotemporal hippocampal axis of adult rats and their modulation by an early life generalized seizure. Epilepsia 53(5):879–887. doi:10.1111/j.1528-1167.2012.03440.x
Sener U, Zorlu Y, Karaguzel O, Ozdamar O, Coker I, Topbas M (2006) Effects of common anti-epileptic drug monotherapy on serum levels of homocysteine, vitamin B12, folic acid and vitamin B6. Seizure 15(2):79–85
Scherer EB, Loureiro SO, Vuaden FC, da Cunha AA, Schmitz F, Kolling J, Savio LE, Bogo MR, Bonan CD, Netto CA, Wyse AT (2014) Mild hyperhomocysteinemia increases brain acetylcholinesterase and proinflammatory cytokine levels in different tissues. Mol Neurobiol. (in press)
Massoulie J, Perrier N, Noureddine H, Liang D, Bon S (2008) Old and new questions about cholinesterases. Chem Biol Interact 175(1–3):30–44. doi:10.1016/j.cbi.2008.04.039
Silman I, Sussman JL (2008) Acetylcholinesterase: How is structure related to function? Chem Biol Interact 175:3–10. doi:10.1016/j.cbi.2008.05.035
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of Serbia (Grant #175032). We are grateful to reviewers for their constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrnčić, D., Rašić -Marković, A., Stojković, T. et al. Hyperhomocysteinemia induced by methionine dietary nutritional overload modulates acetylcholinesterase activity in the rat brain. Mol Cell Biochem 396, 99–105 (2014). https://doi.org/10.1007/s11010-014-2146-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2146-8