Abstract
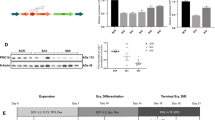
Characterized for the first time in erythrocytes, phosphatidylinositol phosphate kinases (PIP kinases) belong to a family of enzymes that generate various lipid messengers and participate in several cellular processes, including gene expression regulation. Recently, the PIPKIIα gene was found to be differentially expressed in reticulocytes from two siblings with hemoglobin H disease, suggesting a possible relationship between PIPKIIα and the production of globins. Here, we investigated PIPKIIα gene and protein expression and protein localization in hematopoietic-derived cells during their differentiation, and the effects of PIPKIIα silencing on K562 cells. PIPKIIα silencing resulted in an increase in α and γ globins and a decrease in the proliferation of K562 cells without affecting cell cycle progression and apoptosis. In conclusion, using a cell line model, we showed that PIPKIIα is widely expressed in hematopoietic-derived cells, is localized in their cytoplasm and nucleus, and is upregulated during erythroid differentiation. We also showed that PIPKIIα silencing can induce α and γ globin expression and decrease cell proliferation in K562 cells.





Similar content being viewed by others
References
Doughman RL, Firestone AJ, Anderson RA (2003) Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol 194:77–89
Michell RH, Conroy LA, Finney M, French PJ, Bunce CM, Anderson K, Baxter MA, Brown G, Gordon J, Jenkinson EJ et al (1992) Inositol lipids and phosphates in the proliferation and differentiation of lymphocytes and myeloid cells. Ciba Found Symp 164:2–11 discussion 12–16
Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC (1999) Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem 274:9907–9910
Bunce MW, Boronenkov IV, Anderson RA (2008) Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J Biol Chem 283:8678–8686
Clarke JH, Wang M, Irvine RF (2010) Localization, regulation and function of type II phosphatidylinositol 5-phosphate 4-kinases. Adv Enzyme Regul 50:12–18
Ling LE, Schulz JT, Cantley LC (1989) Characterization and purification of membrane-associated phosphatidylinositol-4-phosphate kinase from human red blood cells. J Biol Chem 264:5080–5088
Hinchliffe KA, Irvine RF, Divecha N (1996) Aggregation-dependent, integrin-mediated increases in cytoskeletally associated PtdInsP2 (4,5) levels in human platelets are controlled by translocation of PtdIns 4-P 5-kinase C to the cytoskeleton. EMBO J 15:6516–6524
Wenning MR, Mello MP, Andrade TG, Lanaro C, Albuquerque DM, Saad ST, Costa FF, Sonati MF (2009) PIP4KIIA and beta-globin: transcripts differentially expressed in reticulocytes and associated with high levels of Hb H in two siblings with Hb H disease. Eur J Haematol 83:490–493
Heck JN, Mellman DL, Ling K, Sun Y, Wagoner MP, Schill NJ, Anderson RA (2007) A conspicuous connection: structure defines function for the phosphatidylinositol-phosphate kinase family. Crit Rev Biochem Mol Biol 42:15–39
Boronenkov IV, Loijens JC, Umeda M, Anderson RA (1998) Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell 9:3547–3560
York JD, Odom AR, Murphy R, Ives EB, Wente SR (1999) A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 285:96–100
Bazenet CE, Ruano AR, Brockman JL, Anderson RA (1990) The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem 265:18012–18022
Jenkins GH, Fisette PL, Anderson RA (1994) Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem 269:11547–11554
Boronenkov IV, Anderson RA (1995) The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem 270:2881–2884
Martin TF (2001) PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol 13:493–499
Matsui T, Yonemura S, Tsukita S (1999) Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol 9:1259–1262
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Machado-Neto JA, Favaro P, Lazarini M, Costa FF, Olalla Saad ST, Traina F (2011) Knockdown of insulin receptor substrate 1 reduces proliferation and downregulates Akt/mTOR and MAPK pathways in K562 cells. Biochim Biophys Acta 1813(8):1404–1411
Chang CW, Chou HY, Lin YS, Huang KH, Chang CJ, Hsu TC, Lee SC (2008) Phosphorylation at Ser473 regulates heterochromatin protein 1 binding and corepressor function of TIF1beta/KAP1. BMC Mol Biol 9:61
Di Pietro R, di Giacomo V, Caravatta L, Sancilio S, Rana RA, Cataldi A (2007) Cyclic nucleotide response element binding (CREB) protein activation is involved in K562 erythroleukemia cells differentiation. J Cell Biochem 100:1070–1079
Pass MB, Borregaard N, Cowland JB (2007) Derangement of transcription factor profiles during in vitro differentiation of HL60 and NB4 cells. Leuk Res 31:827–837
Ferreira R, Ohneda K, Yamamoto M, Philipsen S (2005) GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol 25:1215–1227
Stachura DL, Chou ST, Weiss MJ (2006) Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood 107:87–97
Bultsma Y, Keune WJ, Divecha N (2010) PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. Biochem J 430:223–235
Luoh SW, Venkatesan N, Tripathi R (2004) Overexpression of the amplified Pip4k2beta gene from 17q11-12 in breast cancer cells confers proliferation advantage. Oncogene 23:1354–1363
Clarke JH, Richardson JP, Hinchliffe KA, Irvine RF (2007) Type II PtdInsP kinases: location, regulation and function. Biochem Soc Symp 74:149–159
Wang M, Bond NJ, Letcher AJ, Richardson JP, Lilley KS, Irvine RF, Clarke JH (2010) Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIalpha and IIbeta and a partial nuclear localization of the IIalpha isoform. Biochem J 430:215–221
Acknowledgments
The authors would like to thank Tereza Salles for her valuable technical assistance and the National Institute of Science and Technology for Photonics Applied to Cell Biology (INFABIC) for providing access to equipment and assistance. This work received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil.
Conflict of interest
The authors have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peretti de Albuquerque Wobeto, V., Machado-Neto, J.A., Zaccariotto, T.R. et al. PIPKIIα is widely expressed in hematopoietic-derived cells and may play a role in the expression of alpha- and gamma-globins in K562 cells. Mol Cell Biochem 393, 145–153 (2014). https://doi.org/10.1007/s11010-014-2054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2054-y