Abstract
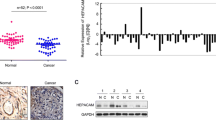
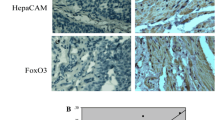
Hepatocyte cell adhesion molecule (HepaCAM) plays a crucial role in tumor progression and has been recognized as a novel tumor suppressor gene. The high protein expression level of protein kinase Cε (PKCε) has been discovered in many tumor types. In the present study, we determined HepaCAM and PKCε protein levels in human clear cell renal cell carcinoma (ccRCC) tissues and analyzed the correlation between them. We observed an inverse relationship in the expression of HepaCAM and PKCε in ccRCC and adjacent normal tissues. In ccRCC tissue, HepaCAM expression was undetectable while PKCε expression was high; the opposite was found in the adjacent normal tissue. Western blot analysis demonstrated that PKCε cytosolic protein levels increased while plasma membrane protein levels decreased without any change in total protein following infection of the ccRCC cell line 786-0 with adenovirus-GFP-HepaCAM (Ad-GFP-HepaCAM). Moreover, the application of Ad-GFP-HepaCAM combined with a PKCε-specific translocation inhibitor (εV1-2) effectively inhibited 786-0 cell growth. Ad-mediated expression of HepaCAM in 786-0 cells reduced the levels of phosphorylated AKT and cyclin D1 and inhibited cell proliferation. In summary, our studies point to interesting connections between HepaCAM and PKCε in tissues and in vitro. HepaCAM may prevent the translocation of PKCε from cytosolic to particulate fractions, resulting in the inhibition of 786-0 cell proliferation. Therapeutic manipulation of these novel protein targets may provide new ways of treating ccRCC.



Similar content being viewed by others
References
Ljungberg B, Cowan NC, Hanbury DC et al (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58:398–406
Staehler M, Haseke N, Schoeppler G et al (2007) Modern therapeutic approaches in metastatic renal cell carcinoma. EAU-EBU Update Ser 5:26–37
Chung Moh M, Hoon Lee L, Shen S (2005) Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma. J Hepatol 42:833–841
Yang S, Wu X, Luo C et al (2010) Expression and clinical significance of hepaCAM and VEGF in urothelial carcinoma. World J Urol 28:473–478
Xun C, Luo C, Wu X et al (2010) Expression of hepaCAM and its effect on proliferation of tumor cells in renal cell carcinoma. Urology 75:828–834
Moh MC, Zhang T, Lee LH et al (2008) Expression of hepaCAM is downregulated in cancers and induces senescence-like growth arrest via a p53/p21-dependent pathway in human breast cancer cells. Carcinogenesis 29:2298–2305
Wang Q, Luo C, Wu X et al (2013) HepaCAM and p-mTOR closely correlate in bladder transitional cell carcinoma and hepaCAM expression inhibits proliferation via an AMPK/mTOR dependent pathway in human bladder cancer cells. J Urol 190:1912–1918
Xu B, He Y, Wu X et al (2012) Exploration of the correlations between interferon-γ in patient serum and HepaCAM in bladder transitional cell carcinoma, and the interferon-γ mechanism inhibiting BIU-87 proliferation. J Urol 188:1346–1353
Lee LH, Moh MC, Zhang T et al (2009) The immunoglobulin-like cell adhesion molecule HepaCAM induces differentiation of human glioblastoma U373-MG cells. J Cell Biochem 107:1129–1138
He Y, Wu X, Luo C et al (2010) Functional significance of the HepaCAM gene in bladder cancer. BMC Cancer 10:83
Zhang QL, Luo CL, Wu XH et al (2011) HepaCAM induces G1 phase arrest and promotes c-Myc degradation in human renal cell carcinoma. J Cell Biochem 112:2910–2919
Hafeez BB, Zhong W, Weichert J et al (2011) Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res 71:2318–2327
Totoń E, Ignatowicz E, Skrzeczkowska K et al (2011) Protein kinase C ε as a cancer marker and target for anticancer therapy. Pharmacol Rep 63:19–29
Bae KM, Wang H, Jiang G et al (2007) Protein kinase C is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res 67:6053–6063
Akita Y (2002) Protein kinase C ε (PKC ε) its unique structure and function. J Bio chem 132:847–852
Kashiwagi K (2002) Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J Biol Chem 277:18037–18045
Csukai M, Chen CH, De Matteis MA et al (1997) The coatomer protein beta ‘-COP, a selective binding protein (RACK) for protein kinase C epsilon. J Biol Chem 272:29200–29206
Liron T, Chen LE, Khaner H et al (2007) Rational design of a selective antagonist of epsilon protein kinase C derived from the selective allosteric agonist, pseudo-RACK peptide. J Mol Cell Cardiol 42:835–841
Diouf B, Collazos A, Labesse G et al (2009) A 20-amino acid module of protein kinase C involved in translocation and selective targeting at cell-cell contacts. J Biol Chem 284:18808–18815
Edge SB, Byrd, Carducci M et al (2009) AJCC cancer staging handbook: from the AJCC cancer staging manual, vol 7. Springer, New York, pp 547–560
Störkel S, Eble JN, Adlakha K et al (1997) Classification of renal cell carcinoma. Cancer 80:987–989
Ruckhäberle E, Karn T, Denkert C et al (2013) Predictive value of sphingosine kinase 1 expression in neoadjuvant treatment of breast cancer. J Cancer Res Clin Oncol 139:1681–1689
England K, Rumsby MG (2000) Changes in protein kinase C epsilon phosphorylation status and intracellular localization as 3T3 and 3T6 fibroblasts grow to confluency and quiescence_ a role for phosphorylation at ser-729? Biochem J 352:19–26
Moh MC, Zhang C, Luo C et al (2005) Structural and functional analyses of a novel Ig-like cell adhesion molecule, HepaCAM, in the human breast carcinoma MCF7 cells. J Biol Chem 280:27366–27374
Brenner W, Benzing F, Gudejko-Thiel J et al (2004) Regulation of beta1 integrin expression by PKC epsilon in renal cancer cells. Int J Oncol 25:1157–1163
Bright R, Sun GH, Yenari MA et al (2008) ε PKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci Lett 441:120–124
Nelson TJ, Cui C, Luo Y et al (2009) Reduction of beta-amyloid levels by novel protein kinase c (epsilon) activators. J Biol Chem 284:34514–34521
Slupsky JR, Kamiguti AS, Harris RJ et al (2007) Central role of protein kinase Cε in constitutive activation of ERK1/2 and Rac1 in the malignant cells of hairy cell leukemia. Am J Pathol 170:745–754
Basu A, Sivaprasad U (2007) Protein kinase Cε makes the life and death decision. Cell Signal 19:1633–1642
Garg R, Blando J, Perez CJ et al (2012) Activation of nuclear factor B (NF-B) in prostate cancer is mediated by protein kinase C epsilon (PKC epsilon). J Biol Chem 287:37570–37582
Aziz MH, Manoharan HT, Church DR et al (2007) Protein kinase C interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res 67:8828–8838
Acknowledgments
The study was done in the Department of Laboratory Diagnosis, Chongqing Medical University.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, B., Tan, J., Du, H. et al. HepaCAM inhibits clear cell renal carcinoma 786-0 cell proliferation via blocking PKCε translocation from cytoplasm to plasma membrane. Mol Cell Biochem 391, 95–102 (2014). https://doi.org/10.1007/s11010-014-1991-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-1991-9