Abstract
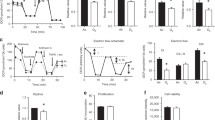
An inspiratory oxygen fraction of 1.0 is often required to avoid hypoxia both in many pre- and in-hospital situations. On the other hand, hyperoxia may lead to deleterious consequences (cell growth inhibition, inflammation, and apoptosis) for numerous tissues including the lung. Whereas clinical effects of hyperoxic lung injury are well known, its impact on the expression of lung proteins has not yet been evaluated sufficiently. The aim of this study was to analyze time-dependent alterations of protein expression in rat lung tissue after short-term normobaric hyperoxia (NH). After approval of the local ethics committee for animal research, N = 36 Wistar rats were randomized into six different groups: three groups with NH with exposure to 100 % oxygen for 3 h and three groups with normobaric normoxia (NN) with exposure to room air (21 % oxygen). After the end of the experiments, lungs were removed immediately (NH0 and NN0), after 3 days (NH3 and NN3) and after 7 days (NH7 and NN7). Lung lysates were analyzed by two-dimensional gel electrophoresis (2D-GE) followed by peptide mass fingerprinting using mass spectrometry. Statistical analysis was performed with Delta 2D (DECODON GmbH, Greifswald, Germany; ANOVA, Bonferroni correction, p < 0.01). Biological functions of differential regulated proteins were studied using functional network analysis (Ingenuity Pathways Analysis, IPA). pO2 was significantly higher in NH-groups compared to NN-groups (581 ± 28 vs. 98 ± 12 mmHg; p < 0.01), all other physiological parameters did not differ. Expression of 14 proteins were significantly altered: two proteins were up-regulated and 12 proteins were down-regulated. Even though NH was comparatively short termed, significant alterations in lung protein expression could be demonstrated up to 7 days after hyperoxia. The identified proteins indicate an association with cell growth inhibition, regulation of apoptosis, and approval of structural cell integrity.



Similar content being viewed by others
References
Halliwell B, Cross C (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Supp. 10):5–12
Bostek CC (1989) Oxygen toxicity: an introduction. AANA J 57(3):231–237
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116(1 Suppl):9S–15S
Waxman AB, Einarsson O, Seres T, Knickelbein RG, Homer R, Warshaw JB, Johnston R, Elias JA (1999) Targeted lung expression of interleukin-11 enhances murine tolerance of 100 % oxygen and diminishes hyperoxia-induced DNA fragmentation. Chest 116(1 Suppl):8S–9S
Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, Waxman AB, Elias JA (2000) IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 106(6):783–791. doi:10.1172/JCI9674
Tang G, White JE, Gordon RJ, Lumb PD, Tsan MF (1993) Polyethylene glycol-conjugated superoxide dismutase protects rats against oxygen toxicity. J Appl Physiol 74(3):1425–1431
Asikainen TM, White CW (2004) Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal 6(1):155–167. doi:10.1089/152308604771978462
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344. doi:10.1113/jphysiol.2003.049478
Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AM, Lee PJ (2003) Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol 28(3):305–315
Tsan MF (2001) Superoxide dismutase and pulmonary oxygen toxicity: lessons from transgenic and knockout mice (review). Int J Mol Med 7(1):13–19
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4(3):181–189. doi:10.1038/nri1312
Freeman BA, Crapo JD (1981) Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 256(21):10986–10992
Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N (2007) Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101(3):258–267. doi:10.1161/CIRCRESAHA.107.148015
Fox RB, Hoidal JR, Brown DM, Repine JE (1981) Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis 123(5):521–523
Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR (1996) Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 154(3 Pt 1):602–611
Raj JU, Hazinski TA, Bland RD (1985) Oxygen-induced lung microvascular injury in neutropenic rabbits and lambs. J Appl Physiol 58(3):921–927
Mantell LL, Horowitz S, Davis JM, Kazzaz JA (1999) Hyperoxia-induced cell death in the lung–the correlation of apoptosis, necrosis, and inflammation. Ann N Y Acad Sci 887:171–180
O’Reilly MA (2001) DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 281(2):L291–L305
Hinkelbein J, Feldmann REJ, Peterka A, Schubert C, Schelshorn D, Maurer MH, Kalenka A (2007) Alterations in cerebral metabolomics and proteomic expression during sepsis. Curr Neurovasc Res 4(4):280–288
Kalenka A, Hinkelbein J, Feldmann RJ, Kuschinsky W, Waschke K, Maurer M (2007) The effects of sevoflurane anesthesia on rat brain proteins: a proteomic time-course analysis. Anesth Analg 104(5):1129–1135
Hinkelbein J, Kalenka A, Feldmann R (2009) Early alterations in rat brain protein expression during sepsis. Anaesthesist 58:134–143
Hinkelbein J, Feldmann RJ, Kalenka A (2010) Time-dependent alterations of cerebral proteins following short-term normobaric hyperoxia. Mol Cell Biochem 339(1):9–21
Bradford M (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(5):248–254
Schlags W, Walther M, Masree M, Kratzel M, Noe C, Lachmann B (2005) Towards validating a method for two-dimensional electrophoresis/silver staining. Electrophoresis 26(12):2461–2469
Maurer M (2005) Comparison of large proteomic datasets. Curr Proteomics 2:179–189
Berth M, Moser FM, Kolbe M, Bernhardt J (2007) The state of the art in the analysis of two-dimensional gel electrophoresis images. Appl Microbiol Biotechnol 76(6):1223–1243. doi:10.1007/s00253-007-1128-0
Perkins D, Pappin D, Creasy D, Cottrell J (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20(18):3551–3567
Gerling I, Singh S, Lenchik N, Marshall D, Wu J (2006) New data analysis and mining approaches identify unique proteome and transcriptome markers of susceptibility to autoimmune diabetes. Mol Cell Proteomics 5(2):293–305
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 5:1609–1623
Hinkelbein J, Feldmann RE Jr, Kalenka A (2010) Time-dependent alterations of cerebral proteins following short-term normobaric hyperoxia. Mol Cell Biochem 339(1–2):9–21. doi:10.1007/s11010-009-0365-1
Fensterl V, Sen GC (2011) The ISG56/IFIT1 gene family. J Interferon Cytokine Res 31(1):71–78. doi:10.1089/jir.2010.0101
Guo J, Hui DJ, Merrick WC, Sen GC (2000) A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J 19(24):6891–6899. doi:10.1093/emboj/19.24.6891
Li Y, Li C, Xue P, Zhong B, Mao AP, Ran Y, Chen H, Wang YY, Yang F, Shu HB (2009) ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci USA 106(19):7945–7950. doi:10.1073/pnas.0900818106
Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8(1):61–70
Bova MP, McHaourab HS, Han Y, Fung BK (2000) Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem 275(2):1035–1042
Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S (2001) Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6(1):49–58
Jakob U, Gaestel M, Engel K, Buchner J (1993) Small heat shock proteins are molecular chaperones. J Biol Chem 268(3):1517–1520
Huot J, Lambert H, Lavoie JN, Guimond A, Houle F, Landry J (1995) Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur J Biochem 227(1–2):416–427
Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154(1):363–374
Narayanan A, LeClaire LL 3rd, Barber DL, Jacobson MP (2011) Phosphorylation of the Arp2 subunit relieves auto-inhibitory interactions for Arp2/3 complex activation. PLoS Comput Biol 7(11):e1002226. doi:10.1371/journal.pcbi.1002226
Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL (2004) Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci 7(2):145–152. doi:10.1038/nn1179nn1179
Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13:83–117. doi:10.1146/annurev.cellbio.13.1.83
Micucci C, Orciari S, Catalano A (2010) Semaphorins and their receptors in stem and cancer cells. Curr Med Chem 17(30):3462–3475
Turano C, Coppari S, Altieri F, Ferraro A (2002) Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol 193(2):154–163. doi:10.1002/jcp.10172
Ellgaard L, Frickel EM (2003) Calnexin, calreticulin, and ERp57: teammates in glycoprotein folding. Cell Biochem Biophys 39(3):223–247. doi:10.1385/CBB:39:3:223
Xu D, Perez RE, Rezaiekhaligh MH, Bourdi M, Truog WE (2009) Knockdown of ERp57 increases BiP/GRP78 induction and protects against hyperoxia and tunicamycin-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 297(1):L44–L51. doi:10.1152/ajplung.90626.2008
Wang G, Zuo X, Liu J, Jiang L, Liu Y, Zheng Y, Zhang B, Xiao X (2009) Expression of mipu1 in response to myocardial infarction in rats. Int J Mol Sci 10(2):492–506. doi:10.3390/ijms10020492
Qu S, Zhu H, Wei X, Zhang C, Jiang L, Liu Y, Luo Q, Xiao X (2010) Oxidative stress-mediated up-regulation of myocardial ischemic preconditioning up-regulated protein 1 gene expression in H9c2 cardiomyocytes is regulated by cyclic AMP-response element binding protein. Free Radic Biol Med 49(4):580–586. doi:10.1016/j.freeradbiomed.2010.05.004
Hay RT (2005) SUMO: a history of modification. Mol Cell 18(1):1–12. doi:10.1016/j.molcel.2005.03.012
Yeh ET (2009) SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem 284(13):8223–8227. doi:10.1074/jbc.R800050200
Sang J, Yang K, Sun Y, Han Y, Cang H, Chen Y, Shi G, Wang K, Zhou J, Wang X, Yi J (2011) SUMO2 and SUMO3 transcription is differentially regulated by oxidative stress in an Sp1-dependent manner. Biochem J 435(2):489–498. doi:10.1042/BJ20101474
Pulimeno P, Bauer C, Stutz J, Citi S (2010) PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-cadherin. PLoS One 5(8):e12207. doi:10.1371/journal.pone.0012207
Meng W, Takeichi M (2009) Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1(6):a002899. doi:10.1101/cshperspect.a002899
Grommes J, Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol Med 17(3–4):293–307. doi:10.2119/molmed.2010.00138
Gilligan DM, Lozovatsky L, Gwynn B, Brugnara C, Mohandas N, Peters LL (1999) Targeted disruption of the beta adducin gene (Add2) causes red blood cell spherocytosis in mice. Proc Natl Acad Sci USA 96(19):10717–10722
Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE (2006) A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J 25(20):4808–4819. doi:10.1038/sj.emboj.7601366
Na N, Chandel NS, Litvan J, Ridge KM (2010) Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J 24(3):799–809. doi:10.1096/fj.08-128967
Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D et al (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270(43):25752–25761
Koo HC, Davis JM, Li Y, Hatzis D, Opsimos H, Pollack S, Strayer MS, Ballard PL, Kazzaz JA (2005) Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J Physiol Lung Cell Mol Physiol 288(4):L718–L726. doi:10.1152/ajplung.00456.2003
Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S (1997) Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem 272(33):20646–20649
Fountoulakis M (2001) Proteomics: current technologies and applications in neurological disorders and toxicology. Amino Acids 21:363–381
Acknowledgments
The technical assistance of M. Harlacher and M. Lorenz (University of Heidelberg) is gratefully acknowledged.
Conflict of interest
OS, WAW, AK, and JH do not have any conflict of interest with any of the companies or products mentioned. GW is employed at DECODON GmbH, Greifswald, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spelten, O., Wetsch, W.A., Wrettos, G. et al. Response of rat lung tissue to short-term hyperoxia: a proteomic approach. Mol Cell Biochem 383, 231–242 (2013). https://doi.org/10.1007/s11010-013-1771-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1771-y