Abstract
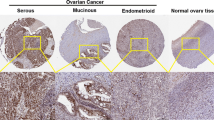
P21-activated kinase 5 (PAK5) is the recently identified member of the group II p21-activated kinases (PAKs) family, which is characterized by a highly conserved amino-terminal Cdc42/Rac interactive binding domain and a carboxyl terminal kinase domain. However, the role of PAK5 in gynecological cancers has not been evaluated so far. It is remarkable that we found PAK5 was overexpressed in epithelial ovarian cancer (EOC), which is faced with an obstacle of paclitaxel resistance. Therefore, in this study, we aim to examine the PAK5 expression during EOC progression, the role of PAK5 in malignant progression of EOC and the probable relationship between PAK5 and EOC paclitaxel resistance. By immunohistochemistry, our results showed that PAK5 expression was increased with EOC progression through the adenoma to carcinoma sequence, with the highest expression level in invasive and metastatic EOCs. Furthermore, the expression level of PAK5 was also found to increase in accordance with the development of EOC Federation International of Gynecology and Obstetrics stages (P = 0.038) and differentiation grades (P = 0.008). Remarkably, those patients who recurred within 6 months after accepting tumor reductive surgery and the following carboplatin + paclitaxel chemotherapy had the highest PAK5 expression (P = 0.015). Moreover, in in vitro studies, we found that SK-OV-3 cell growth was decreased while paclitaxel chemosensitivity was correspondingly increased with the down-regulation of PAK5. Taken together, our study demonstrated that PAK5 is correlated to human EOC and increased PAK5 expression promotes EOC progression, and PAK5 regulates EOC cell paclitaxel chemoresistance.




Similar content being viewed by others
References
Cotteret S, Jaffer ZM, Beeser A, Chernoff J (2003) p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol 23(16):5526–5539
Wang X, Gong W, Qing H, Geng Y, Wang X, Zhang Y et al (2010) p21-activated kinase 5 inhibits camptothecin-induced apoptosis in colorectal carcinoma cells. Tumour Biol 31(6):575–582
Cau J, Faure S, Comps M, Delsert C, Morin N (2001) A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J Cell Biol 155(6):1029–1042
Nguyen TV, Galvan V, Huang W, Banwait S, Tang H, Zhang J et al (2008) Signal transduction in Alzheimer disease: p21-activated kinase signaling requires C-terminal cleavage of APP at Asp664. J Neurochem 104(4):1065–1080
Eswaran J, Lee WH, Debreczeni JE, Filippakopoulos P, Turnbull A, Fedorov O et al (2007) Crystal structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure 15(2):201–213
Dan I, Watanabe NM, Kusumi A (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 11(5):220–230
Cotteret S, Chernoff J (2006) Nucleocytoplasmic shuttling of Pak5 regulates its antiapoptotic properties. Mol Cell Biol 26(8):3215–3230
Dan C, Nath N, Liberto M, Minden A (2002) PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol 22(2):567–577
Salminen A, Suuronen T, Kaarniranta K (2008) ROCK, PAK, and Toll of synapses in Alzheimer’s disease. Biochem Biophys Res Commun 371(4):587–590
Timm T, Matenia D, Li XY, Griesshaber B, Mandelkow EM (2006) Signaling from MARK to tau: regulation, cytoskeletal crosstalk, and pathological phosphorylation. Neurodegener Dis 3(4–5):207–217
Permuth-Wey J, Sellers TA (2009) Epidemiology of ovarian cancer. Methods Mol Biol 472:413–437
Vasey PA (2003) Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br J Cancer 89(Suppl 3):S23–S28
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93(9):2325–2327
Detre S, Saclani Jotti G, Dowsett M (1995) A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 48(9):876–878
Lopez-Gasco P, Iglesias I, Benedi J, Lozano R, Blanco MD (2012) Characterization and in vitro bioactivity evaluation of paclitaxel-loaded polyester nanoparticles. Anticancer Drugs 23(9):947–958
Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK et al (2004) p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem 279(2):1422–1428
Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G (2006) Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst 98(10):671–680
Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO et al (2004) Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res 10(10):3448–3456
Wells CM, Abo A, Ridley AJ (2002) PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J Cell Sci 115(Pt 20):3947–3956
Matenia D, Griesshaber B, Li XY, Thiessen A, Johne C, Jiao J et al (2005) PAK5 kinase is an inhibitor of MARK/Par-1, which leads to stable microtubules and dynamic actin. Mol Biol Cell 16(9):4410–4422
Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E et al (2002) Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21(24):3939–3948
Giroux V, Iovanna JL, Garcia S, Dagorn JC (2009) Combined inhibition of PAK7, MAP3K7 and CK2alpha kinases inhibits the growth of MiaPaCa2 pancreatic cancer cell xenografts. Cancer Gene Ther 16(9):731–740
Brigulova K, Cervinka M, Tosner J, Sedlakova I (2010) Chemoresistance testing of human ovarian cancer cells and its in vitro model. Toxicol In Vitro 24(8):2108–2115
Salom E, Penalver M, Homesley H, Burrell M, Garrett A, Presant CA et al (2012) Correlation of pretreatment drug induced apoptosis in ovarian cancer cells with patient survival and clinical response. J Transl Med 10(1):162
Wu X, Carr HS, Dan I, Ruvolo PP, Frost JA (2008) p21 activated kinase 5 activates Raf-1 and targets it to mitochondria. J Cell Biochem 105(1):167–175
Acknowledgments
The authors thank Dr YinBin Liu (Department of Surgery, Xinhua Hospital, Shanghai, China) and XinYuan Lao (Hollybio, Shanghai, China) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Yao, X. & Zhang, P. The overexpression of P21-activated kinase 5 (PAK5) promotes paclitaxel-chemoresistance of epithelial ovarian cancer. Mol Cell Biochem 383, 191–199 (2013). https://doi.org/10.1007/s11010-013-1767-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1767-7