Abstract
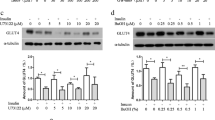
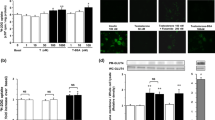
Phosphatidylinositol-3,4,5-triphosphate (PIP3) and phosphatidylinositol-4,5-biphosphate (PIP2) are two well-known membrane bound polyphosphoinositides. Diabetes is associated with impaired glucose metabolism. Using a 3T3L1 adipocyte cell model, this study investigated the role of PIP3 and PIP2 on insulin stimulated glucose metabolism in high glucose (HG) treated cells. Exogenous PIP3 supplementation (1, 5, or 10 nM) increased the phosphorylation of AKT and PKCζ/λ, which in turn upregulated GLUT4 total protein expression as well as its surface expression, glucose uptake, and glucose utilization in cells exposed to HG (25 mM); however, PIP2 had no effect. Comparative signal silencing studies with antisense AKT2 and antisense PKCζ revealed that phosphorylation of PKCζ/λ is more effective in PIP3 mediated GLUT4 activation and glucose utilization than in AKT phosphorylation. Supplementation with PIP3 in combination with insulin enhanced glucose uptake and glucose utilization compared to PIP2 with insulin, or insulin alone, in HG-treated adipocytes. This suggests that a decrease in cellular PIP3 levels may cause impaired insulin sensitivity in diabetes. PIP3 supplementation also prevented HG-induced MCP-1 and resistin secretion and lowered adiponectin levels. This study for the first time demonstrates that PIP3 but not PIP2 plays an important role in GLUT4 upregulation and glucose metabolism mediated by AKT/PKCζ/λ phosphorylation. Whether PIP3 levels in blood can be used as a biomarker of insulin resistance in diabetes needs further investigation.







Similar content being viewed by others
References
Pendaries C, Tronchère H, Plantavid M, Payrastre B (2003) Phosphoinositide signaling disorders in human diseases. FEBS Lett 546:25–31
Czech MP (2000) PIP2 and PIP3: complex roles at the cell surface. Cell 100:603–606
Hinchliffe KA (2001) Cellular signalling: stressing the importance of PIP3. Curr Biol 11:R371–R3373
Park WS, Heo WD, Whalen JH, O’Rourke NA, Bryan HM, Meyer T, Teruel MN (2008) Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell 30:381–392
Várnai P, Bondeva T, Tamás P, Tóth B, Buday L, Hunyady L, Balla T (2005) Selective cellular effects of overexpressed pleckstrin-homology domains that recognize PtdIns(3,4,5)P3 suggest their interaction with protein binding partners. J Cell Sci 118:4879–4888
Toker A (1998) The synthesis and cellular roles of phosphatidylinositol-4,5-bisphosphate. Curr Opin Cell Biol 10:254–261
Taniguchi CM, Tran TT, Kondo T et al (2006) Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc Natl Acad Sci USA 103:12093–12097
Butler M, McKay RA, Popoff IJ et al (2002) Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes 51:1028–1034
Ono H, Katagiri H, Funaki M et al (2001) Regulation of phosphoinositide metabolism, AKT phosphorylation, and glucose transport by PTEN (phosphatase and tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol Endocrinol 15:1411–1422
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Manna P, Jain SK (2011) Hydrogen sulfide and l-cysteine increase phosphatidylinositol-3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3L1 adipocytes. J Biol Chem 286:39848–39859
Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O (1995) Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Influence of ketone bodies. Diabetes Care 18:549–551
Jung DW, Ha HH, Zheng X, Chang YT, Williams DR (2011) Novel use of fluorescent glucose analogues to identify a new class of triazine-based insulin mimetics possessing useful secondary effects. Mol Biosyst 7:346–358
Koshy S, Alizadeh P, Timchenko LT, Beeton C (2010) Quantitative measurement of GLUT4 translocation to the plasma membrane by flow cytometry. J Vis Exp 45:e2429
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Sasaki T, Sasaki J, Sakai T, Takasuga S, Suzuki A (2007) The physiology of phosphoinositides. Biol Pharm Bull 30:1599–1604
Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV (2000) Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology 141:4120–4127
Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV (1997) Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology 138:4721–4731
Bandyopadhyay G, Standaert ML, Zhao L et al (1997) Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem 272:2551–2558
Kotani K, Ogawa W, Matsumoto M et al (1998) Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol 18:6971–6982
Farese RV (2002) Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab 283:E1–E11
Olson AL, Pessin JE (1996) Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 16:235–256
Jiang T, Sweeney G, Rudolf MT, Klip A, Traynor-Kaplan A, Tsien RY (1998) Membrane-permeant esters of phosphatidylinositol-3,4,5-trisphosphate. J Biol Chem 273:11017–11024
Funaki M, DiFransico L, Janmey PA (2006) PI-4,5-P2 stimulates glucose transport activity of GLUT4 in the plasma membrane of 3T3-L1 adipocytes. Biochim Biophys Acta 1763:889–899
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM et al (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312
Egashira K (2003) Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension 41:834–841
Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A et al (2003) Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes 52:1926–1934
Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB (2003) Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes 52:1935–1942
Xiao H, Liu M (2012) Atypical protein kinase C in cell motility. Cell Mol Life Sci (in press)
Hirai T, Chida K (2003) Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133:1–7
Beeson M, Sajan MP, Daspet JG, Luna V, Dizon M, Grebenev D et al (2004) Defective activation of protein kinase C–z in muscle by insulin and phosphatidylinositol-3,4,5,-(PO(4))(3) in obesity and polycystic ovary syndrome. Metab Syndr Relat Disord 2:49–56
Vollenweider P, Menard B, Nicod P (2002) Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol- 3-kinase activation, and impaired atypical protein kinase C (ζ/λ) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes 15:1052–1059
Luna V, Casauban L, Sajan MP, Gomez-Daspet J, Powe JL, Miura A et al (2006) Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia 49:375–382
Acknowledgments
The authors thank Ms. Georgia Morgan for excellent editing of this manuscript. The authors were supported by Grants from NIDDK and the Office of Dietary Supplements of the National Institutes of Health RO1 DK072433 and the Malcolm Feist Endowed Chair in Diabetes. This study was also funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center, Shreveport.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manna, P., Jain, S.K. PIP3 but not PIP2 increases GLUT4 surface expression and glucose metabolism mediated by AKT/PKCζ/λ phosphorylation in 3T3L1 adipocytes. Mol Cell Biochem 381, 291–299 (2013). https://doi.org/10.1007/s11010-013-1714-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1714-7