Abstract
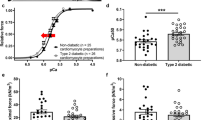
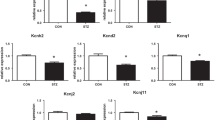
Although, several novel forms of intervention aiming at newly identified therapeutic targets are currently being developed for diabetes mellitus (DM), it is well established that physical exercise continues to be one of the most valuable forms of non-pharmacological therapy. The aim of the study was to investigate the effects of exercise training on excitation–contraction coupling and related gene expression in the Goto-Kakizaki (GK) type 2 diabetic rat heart and whether exercise is able to reverse diabetes-induced changes in excitation–contraction coupling and gene expression. Experiments were performed in GK and control rats aged 10–11 months following 2–3 months of treadmill exercise training. Shortening, [Ca2+]i and L-type Ca2+ current were measured in ventricular myocytes with video edge detection, fluorescence photometry and whole cell patch clamp techniques, respectively. Expression of mRNA was assessed in ventricular muscle with real-time RT-PCR. Amplitude of shortening, Ca2+ transients and L-type Ca2+ current were not significantly altered in ventricular myocytes from GK sedentary compared to control sedentary rats or by exercise training. Expression of mRNA encoding Tpm2, Gja4, Atp1b1, Cacna1g, Cacnb2, Hcn2, Kcna3 and Kcne1 were up-regulated and Gja1, Kcnj2 and Kcnk3 were down-regulated in hearts of sedentary GK rats compared to sedentary controls. Gja1, Cav3 and Kcnk3 were up-regulated and Hcn2 was down-regulated in hearts of exercise trained GK compared to sedentary GK controls. Ventricular myocyte shortening and Ca2+ transport were generally well preserved despite alterations in the profile of expression of mRNA encoding a variety of cardiac muscle proteins in the adult exercise trained GK diabetic rat heart.




Similar content being viewed by others
References
Zimmet PZ, Alberti KG (2006) Introduction: globalization and the non-communicable disease epidemic. Obesity (Silver Spring) 14(1):1–3
Malik M, Bakir A, Saab BA, King H (2005) Glucose intolerance and associated factors in the multi-ethnic population of the United Arab Emirates: results of a national survey. Diabetes Res Clin Pract 69(2):188–195
Julien J (1997) Cardiac complications in non-insulin-dependent diabetes mellitus. J Diabetes Complications 11:123–130
Chareonthaitawee P, Sorajja P, Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ (2007) Prevalence and prognosis of left ventricular systolic dysfunction in asymptomatic diabetic patients without known coronary artery disease referred for stress single-photon emission computed tomography and assessment of left ventricular function. Am Heart J 154(3):567–574
Dounis V, Siegmund T, Hansen A, Jensen J, Schumm-Draeger PM, von Bibra H (2006) Global myocardial perfusion and diastolic function are impaired to a similar extent in patients with type 2 diabetes mellitus and in patients with coronary artery disease—evaluation by contrast echocardiography and pulsed tissue Doppler. Diabetologia 49(11):2729–2740
Loimaala A, Groundstroem K, Majahalme S, Nenonen A, Vuori I (2006) Impaired myocardial function in newly onset type 2 diabetes associates with arterial stiffness. Eur J Echocardiogr 7(5):341–347
Annonu AK, Fattah AA, Mokhtar MS, Ghareeb S, Elhendy A (2001) Left ventricular systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitus. J Am Soc Echocardiogr 14(9):885–891
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87(3):320–323
Di Bonito P, Cuomo S, Moio N, Sibilio G, Sabatini D, Quattrin S, Capaldo B (1996) Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabet Med 13(4):321–324
Hiramatsu K, Ohara N, Shigematsu S, Aizawa T, Ishihara F, Niwa A, Yamada T, Naka M, Momose A, Yoshizawa K (1992) Left ventricular filling abnormalities in non-insulin-dependent diabetes mellitus and improvement by a short-term glycemic control. Am J Cardiol 70(13):1185–1189
Yasuda I, Kawakami K, Shimada T, Tanigawa K, Murakami R, Izumi S, Morioka S, Kato Y, Moriyama K (1992) Systolic and diastolic left ventricular dysfunction in middle-aged asymptomatic non-insulin-dependent diabetics. J Cardiol 22(2–3):427–438
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16 Suppl 1:3–63
Chipkin SR, Klugh SA, Chasan-Taber L (2001) Exercise and diabetes. Cardiol Clin 19(3):489–505
Howarth FC, Almugaddum FA, Qureshi MA, Ljubisavljevic M (2009) The effects of heavy long-term exercise on ventricular myocyte shortening and intracellular Ca(2+) in streptozotocin-induced diabetic rat. J Diabetes Complications 24(4):278–285
Howarth FC, Almugaddum FA, Qureshi MA, Ljubisavijevic M (2008) Effects of varying intensity exercise on shortening and intracellular calcium in ventricular myocytes from streptozotocin (STZ)-induced diabetic rats. Mol Cell Biochem 317(1–2):161–167
Howarth FC, Qureshi MA, Hassan Z, Al Kury LT, Isaev D, Parekh K, Yammahi SR, Oz M, Adrian TE, Adeghate E (2011) Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp Physiol 96(3):325–337
Salem KA, Adrian TE, Qureshi MA, Parekh KA, Oz M, Howarth FC (2012) Shortening and intracellular Ca2+ in ventricular myocytes and expression of genes encoding cardiac muscle proteins in early onset type 2 diabetic Goto-Kakizaki rats. Exp Physiol 97(12):1281–1291
Schrijvers BF, De Vriese AS, Van d V, Rasch R, Lameire NH, Flyvbjerg A (2004) Long-term renal changes in the Goto-Kakizaki rat, a model of lean type 2 diabetes. Nephrol Dial Transplant 19(5):1092–1097
Grijalva J, Hicks S, Zhao X, Medikayala S, Kaminski PM, Wolin MS, Edwards JG (2008) Exercise training enhanced myocardial endothelial nitric oxide synthase (eNOS) function in diabetic Goto-Kakizaki (GK) rats. Cardiovasc Diabetol 19(7):34. doi:10.1186/1475-2840-7-34
Howarth FC, Jacobson M, Shafiullah M, Adeghate E (2008) Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp Physiol 93(3):362–369
Marttila M, Lemola E, Wallefeld W, Memo M, Donner K, Laing NG, Marston S, Gronholm M, Wallgren-Pettersson C (2012) Abnormal actin binding of aberrant beta-tropomyosins is a molecular cause of muscle weakness in TPM2-related nemaline and cap myopathy. Biochem J 442(1):231–239
Delmar M, Makita N (2012) Cardiac connexins, mutations and arrhythmias. Curr Opin Cardiol 27(3):236–241
Meens MJ, Pfenniger A, Kwak BR (2012) Risky communication in atherosclerosis and thrombus formation. Swiss Med Wkly 142:w13553. doi:10.4414/smw.2012.13553.:w13553
Barwe SP, Jordan MC, Skay A, Inge L, Rajasekaran SA, Wolle D, Johnson CL, Neco P, Fang K, Rozengurt N, Goldhaber JI, Roos KP, Rajasekaran AK (2009) Dysfunction of ouabain-induced cardiac contractility in mice with heart-specific ablation of Na, K-ATPase beta1-subunit. J Mol Cell Cardiol 47(4):552–560
Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P (2006) Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98(11):1422–1430
Meissner M, Weissgerber P, Londono JE, Prenen J, Link S, Ruppenthal S, Molkentin JD, Lipp P, Nilius B, Freichel M, Flockerzi V (2011) Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J Biol Chem 286(18):15875–15882
Herrmann S, Layh B, Ludwig A (2011) Novel insights into the distribution of cardiac HCN channels: an expression study in the mouse heart. J Mol Cell Cardiol 51(6):997–1006
Xia S, Wang Y, Zhang Y, Deng SB, Du JL, Wang XC, She Q (2010) Dynamic changes in HCN2, HCN4, KCNE1, and KCNE2 expression in ventricular cells from acute myocardial infarction rat hearts. Biochem Biophys Res Commun 395(3):330–335
Manabe I, Tsuboi M, Ahmmed GU, Sasaki N, Ohtahara A, Yamamoto Y, Hiroe K, Yoshida A, Hisatome I, Shigemasa C (1998) Expression of Shaker-type voltage-gated potassium channel genes in the guinea-pig. Res Commun Mol Pathol Pharmacol 99(1):33–40
Brahmajothi MV, Morales MJ, Rasmusson RL, Campbell DL, Strauss HC (1997) Heterogeneity in K+ channel transcript expression detected in isolated ferret cardiac myocytes. Pacing Clin Electrophysiol 20(2 Pt 2):388–396
Liu Z, Du L, Li M (2012) Update on the slow delayed rectifier potassium current (I(Ks)): role in modulating cardiac function. Curr Med Chem 19(9):1405–1420
Guo J, Wang T, Yang T, Xu J, Li W, Fridman MD, Fisher JT, Zhang S (2011) Interaction between the cardiac rapidly (IKr) and slowly (IKs) activating delayed rectifier potassium channels revealed by low K+ -induced hERG endocytic degradation. J Biol Chem 286(40):34664–34674
Grunnet M (2010) Repolarization of the cardiac action potential. Does an increase in repolarization capacity constitute a new anti-arrhythmic principle? Acta Physiol (Oxf) 198 Suppl 676:1–48
Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R (2002) Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110(3):381–388
Gierten J, Ficker E, Bloehs R, Schweizer PA, Zitron E, Scholz E, Karle C, Katus HA, Thomas D (2010) The human cardiac K2P3.1 (TASK-1) potassium leak channel is a molecular target for the class III antiarrhythmic drug amiodarone. Naunyn Schmiedebergs Arch Pharmacol 381(3):261–270
Besana A, Barbuti A, Tateyama MA, Symes AJ, Robinson RB, Feinmark SJ (2004) Activation of protein kinase C epsilon inhibits the two-pore domain K+ channel, TASK-1, inducing repolarization abnormalities in cardiac ventricular myocytes. J Biol Chem 279(32):33154–33160
Acknowledgments
This work was supported by grants from UAE University and Emirates Foundation. Research in our laboratory is also supported by LABCO a partner of Sigma-Aldrich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salem, K.A., Qureshi, M.A., Sydorenko, V. et al. Effects of exercise training on excitation–contraction coupling and related mRNA expression in hearts of Goto-Kakizaki type 2 diabetic rats. Mol Cell Biochem 380, 83–96 (2013). https://doi.org/10.1007/s11010-013-1662-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1662-2