Abstract
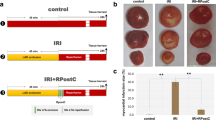
Cardiac oxidative stress is developed following myocardial infarction (MI) particularly in the first week of MI. The influence of reactive oxygen species (ROS) on gene expression profiling and molecular pathways in the infarcted myocardium remains uncertain and is explored in the present study. Rats with MI were treated with or without antioxidants for 1 week. Normal rats served as controls. Cardiac oxidative stress and gene profiling were investigated. Compared to normal hearts, malondialdehyde, a marker of oxidative stress, was significantly increased in the infarcted myocardium, which was significantly suppressed by antioxidants. Microarray assay showed that over a thousand genes were differentially expressed in the infarcted myocardium. Antioxidants significantly altered the expression of 159 genes compared to untreated MI rats. Ingenuity pathway analysis indicated that multiple pathway networks were affected by antioxidants, including those related to cell movement, growth/development, death, and inflammatory/fibrotic responses. IPA further identified that these changes were primarily related to NFκB, p38 MAPK, and ERκ1/2 pathways. Hub genes were identified in the associated gene networks. This study reveals the gene networks associated with cardiac oxidative stress postMI. These observations indicate that ROS regulate various molecular and cellular actions related to cardiac repair/remodeling through multiple gene networks.






Similar content being viewed by others
References
Gorlach A, Kietzmann T, Hess J (2002) Redox signaling through NADPH oxidases: involvement in vascular proliferation and coagulation. Ann N Y Acad Sci 973:505–507
Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR (2002) A NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol 282:C1212–C1224
Nakagami H, Takemoto M, Liao JK (2003) NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 35:851–859
Sorescu D, Griendling K (2002) Reactive oxygen species, mitochondria, and NADPH oxidases in the development and progression of heart failure. Congest Heart Fail 8:132–140
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–52
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:14S–22S
Finkel T (1999) Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukoc Biol 65:337–340
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181–H2190
Seddon M, Looi YH, Shah AM (2007) Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93:903–907
Li L, Quinn MT, Sun Y (2004) Oxidative stress in the infarcted heart: role of de novo angiotensin II production. Biochem Biophys Res Commun 325:943–951
Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y (2001) Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun 281:1200–1206
Grieve DJ, Byrne JA, Cave AC, Shah AM (2004) Role of oxidative stress in cardiac remodeling after myocardial infarction. Heart Lung Circ 13:132–138
Khaper N, Kaur K, Li T, Farahmand F, Singal PK (2003) Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem 251:9–15
Usal A, Acarturk E, Yuregir GT, Unlukurt I, Demirci C, Kurt HI, Birand A (1996) Decreased glutathione levels in acute myocardial infarction. Jpn Heart J 37:177–182
Hare JM (2001) Oxidative stress and apoptosis in heart failure progression. Circ Res 89:198–201
Li WG, Coppey L, Weiss RM, Oskarsson HJ (2001) Antioxidant therapy attenuates JNK activation and apoptosis in the remote noninfarcted myocardium after large myocardial infarction. Biochem Biophys Res Commun 280:353–357
Schoonbroodt S, Piette J (2000) Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol 15:1075–1083
Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL (2002) Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol 39:148–156
Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A (2004) Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 109:544–549
Sun Y, Weber KT (1996) Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol 28:851–858
Wu J, Lenchik NI, Gerling IC (2008) Approaches to reduce false positives and false negatives in the analysis of microarray data: applications in type 1 diabetes research. BMC Genomics 16:S12
Sun Y, Weber KT (2000) Infarct scar: a dynamic tissue. Cardiovasc Res 46:250–256
Daskalopoulos EP, Janssen BJ, Blankesteijn WM (2012) Myofibroblasts in the infarct area: concepts and challenges. Microsc Microanal 18:35–49
Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y (2009) Reactive oxygen species promote angiogenesis in the infarcted rat heart. Int J Exp Pathol 90:621–629
Sun Y (2009) Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81:482–490
Hori M, Nishida K (2009) Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc Res 81:457–464
Lu L, Chen SS, Zhang JQ, Ramires FJ, Sun Y (2004) Activation of nuclear factor-kappaB and its proinflammatory mediator cascade in the infarcted rat heart. Biochem Biophys Res Commun 321:879–885
Nichols TC (2004) NF-kappaB and reperfusion injury. Drug News Perspect 17:99–104
Jacobs M, Staufenberger S, Gergs U, Meuter K, Brandstatter K, Hafner M, Ertl G, Schorb W (1999) Tumor necrosis factor-alpha at acute myocardial infarction in rats and effects on cardiac fibroblasts. J Mol Cell Cardiol 31:1949–1959
Lee KS, Buck M, Houglum K, Chojkier M (1995) Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96:2461–2468
Miyazaki T, Karube M, Matsuzaki Y, Ikegami T, Doy M, Tanaka N, Bouscarel B (2005) Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J Hepatol 43:117–125
Murrell AC, Francis JO, Bromley L (1993) Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 265:659–665
Scarpino S, Marchitti S, Stanzione R, Evangelista A, Di Castro S, Savoia C, Quarta G, Sciarretta S, Ruco L, Volpe M, Rubattu S (2009) Reactive oxygen species-mediated effects on vascular remodeling induced by human atrial natriuretic peptide T2238C molecular variant in endothelial cells in vitro. J Hypertens 27:1804–1813
Iglesias-De La Cruz MC, Ruiz-Torres P, Alcamí J, Díez-Marqués L, Ortega-Velázquez R, Chen S, Rodríguez-Puyol M, Ziyadeh FN, Rodríguez-Puyol D (2001) Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int 59:87–95
Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA (2010) Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod 82:341–351
Ball AM, Sole MJ (1998) Oxidative stress and the pathogenesis of heart failure. Cardiol Clin 16:665–675
Acknowledgments
This work was supported by NIH Heart, Blood, and Lung Institute (RO1-HL096503, Yao Sun).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, W., Zhao, T., Chen, Y. et al. Modification of oxidative stress on gene expression profiling in the rat infarcted heart. Mol Cell Biochem 379, 243–253 (2013). https://doi.org/10.1007/s11010-013-1646-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1646-2