Abstract
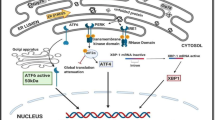
Red-eared slider turtles, Trachemys scripta elegans, can survive for several weeks without oxygen when submerged in cold water. We hypothesized that anaerobiosis is aided by adaptive up-regulation of the unfolded protein response (UPR), a stress-responsive pathway that is activated by accumulation of unfolded proteins in the endoplasmic reticulum (ER) and functions to restore ER homeostasis. RT-PCR, western immunoblotting and DNA-binding assays were used to quantify the responses and/or activation status of UPR-responsive genes and proteins in turtle tissues after animal exposure to 5 or 20 h of anoxic submergence at 4 °C. The phosphorylation state of protein kinase-like ER kinase (PERK) (a UPR-regulated kinase) and eukaryotic initiation factor 2 (eIF2α) increased by 1.43–2.50 fold in response to anoxia in turtle heart, kidney, and liver. Activation of the PERK-regulated transcription factor, activating transcription factor 4 (ATF4), during anoxia was documented by elevated atf4 transcripts and total ATF4 protein (1.60–2.43 fold), increased nuclear ATF4 content, and increased DNA-binding activity (1.44–2.32 fold). ATF3 and GADD34 (downstream targets of ATF4) also increased by 1.38–3.32 fold in heart and liver under anoxia, and atf3 transcripts were also elevated in heart. Two characteristic chaperones of the UPR, GRP78, and GRP94, also responded positively to anoxia with strong increases in both the transcript and protein levels. The data demonstrate that the UPR is activated in turtle heart, kidney, and liver in response to anoxia, suggesting that this pathway mediates an integrated stress response to protect tissues during oxygen deprivation.









Similar content being viewed by others
References
Storey KB (1996) Metabolic adaptations supporting anoxia tolerance in reptiles: recent advances. Comp Biochem Physiol B Biochem Mol Biol 113(1):23–35
Storey KB (2007) Anoxia tolerance in turtles: metabolic regulation and gene expression. Comp Biochem Physiol A Mol Integr Physiol 147(2):263–276
Ultsch GR (2006) The ecology of overwintering among turtles: where turtles overwinter and its consequences. Biol Rev Camb Philos Soc 81(3):339–367
Herbert CV, Jackson DC (1985) Temperature effects on the response to prolonged submergence in the turtle Chrysemys picta bellii. II. Metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol Zool 58:670–681
Hochachka PW, Lutz PL (2001) Mechanism, origin, and evolution of anoxia tolerance in animals. Comp Biochem Physiol B Biochem Mol Biol 130B:435–459
Jackson DC (1968) Metabolic depression and oxygen depletion in the diving turtle. J Appl Physiol 24:503–509
Krivoruchko A, Storey KB (2010) Forever young: mechanisms of natural anoxia tolerance and potential links to longevity. Oxid Med Cell Longev 3(3):186–198
Krivoruchko A, Storey KB (2010) Regulation of the heat shock response under anoxia in the turtle, Trachemys scripta elegans. J Comp Physiol B 180(3):403–414
Prentice HM, Milton SL, Scheurle D, Lutz PL (2004) The upregulation of cognate and inducible heat shock proteins in the anoxic turtle brain. J Cereb Blood Flow Metab 24(7):826–828
Schröder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65(6):862–894
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmicreticulum-resident kinase. Nature 397:271–274
Shi Y, An J, Liang J, Hayes S, Sandusky GE, Stramm LE, Yang NN (1999) Characterization of a mutant pancreatic eIF-2a kinase, PEK, and co-localization with somatostatin in islet delta cells. J Biol Chem 274:5723–5730
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18:7499–7509
Dorner AJ, Wasley LC, Kaufman RJ (1992) Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J 11:1563–1571
Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J (1988) The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464
Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T (2000) A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol 2:379–384
Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K (2006) Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 172:383–393
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101:249–258
Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4:265–271
Sidrauski C, Walter P (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90(6):1031–1039
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20:6755–6767
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1381–1388
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11(5):1475–1489
Krivoruchko A, Storey KB (2010) Molecular mechanisms of turtle anoxia tolerance: a role for NF-kappaB. Gene 450(1–2):63–69
Krivoruchko A, Storey KB (2010) Activation of antioxidant defenses in response to freezing in freeze-tolerant painted turtle hatchlings. Biochim Biophys Acta 1800:662–668
Wek RC, Cavener DR (2007) Translational control and the unfolded protein response. Antioxid Redox Signal 9(12):2357–2371
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Schröder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569(1–2):29–63
Jackson DC, Ultsch GR (2010) Physiology of hibernation under the ice by turtles and frogs. J Exp Zool A Ecol Genet Physiol 313(6):311–327. doi:10.1002/jez.603
Warren DE, Jackson DC (2008) Lactate metabolism in anoxic turtles: an integrative review. J Comp Physiol B 178(2):133–148. doi:10.1007/s00360-007-0212-1
Jackson DC (2004) Acid-base balance during hypoxic hypometabolism: selected vertebrate strategies. Respir Physiol Neurobiol 141(3):273–283. doi:10.1016/j.resp200401009
Fels DR, Koumenis C (2006) The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther 5(7):723–728
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Ameri K, Hammond EM, Culmsee C, Raida M, Katschinski DM, Wenger RH, Wagner E, Davis RJ, Hai T, Denko N, Harris AL (2007) Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene 26(2):284–289
Vatten KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101(31):11269–11274
Hai T (2006) The ATF transcription factors in cellular adaptive responses. In: Ma J (ed) Gene expression and regulation. Higher Education Press, Beijing, pp 322–333
Lu D, Wolfgang CD, Hai T (2006) Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem 281(15):10473–10481
Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153(5):1011–1022
Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol 21(20):6841–6850
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26(8):504–510
Acknowledgments
We thank Jan Storey for editorial review of this manuscript. This research was supported by a discovery grant from the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krivoruchko, A., Storey, K.B. Activation of the unfolded protein response during anoxia exposure in the turtle Trachemys scripta elegans . Mol Cell Biochem 374, 91–103 (2013). https://doi.org/10.1007/s11010-012-1508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1508-3