Abstract
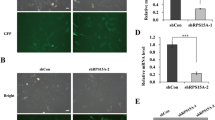
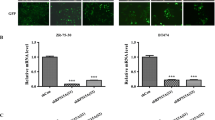
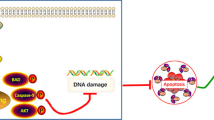
Oncogenic KRAS, an important target for antitumor therapy, contributes to pancreatic cancer tumorigenesis, progression and maintenance. However, intracellular compensation regulation can help cells to resist the targeted therapy. Gene knockdown method such as RNAi may help to understand this intracellular regulatory system and discover novel therapeutic approach. In this study, two stable transfected cell lines were constructed through lentivirus-mediated shRNA targeting KRAS of PANC-1 cells, to investigate cell response to the knockdown of KRAS. Human whole genome microarray was then used to compare the gene expression profile. As a result, ribosomal proteins L26 and L29 (RPL26 and RPL29) were dramatically upregulated by KRAS-shRNA specifically. To identify whether RPL26 or RPL29 was critical for PANC-1 cells, siRNAs against RPL26 and RPL29 were designed and transfected in vitro. The results showed that knockdown of RPL26 or RPL29 expression significantly suppressed cell proliferation, induced cell arrest at G0/G1 phase and enhanced cell apoptosis. Reactive oxygen species (ROS) assay indicated that silencing of RPL26 or RPL29 markedly decreased the intracellular ROS generation. Our findings imply that siRNA interference against RPL26 and RPL29 is of potential value for intervention of pancreatic cancer.







Similar content being viewed by others
Abbreviations
- RNAi:
-
RNA interference
- shRNA:
-
Short hairpin RNA
- siRNA:
-
Small interfering RNA
- ROS:
-
Reactive oxygen species
- PC:
-
Pancreatic cancer
- FBS:
-
Fetal bovine serum
- ACDU:
-
Automated cell deposition unit
- FCM:
-
Flow cytometry
- NC:
-
Negative control
- qPCR:
-
Quantitative real-time PCR
- FITC:
-
Fluorescein isothiocyanate
- PI:
-
Propidium iodide
- PBS:
-
Phosphate-buffered saline
- DCFH-DA:
-
2,7-Dichlorfluorescein-diacetate
- DCF:
-
2,7-Dichlorfluorescein
- MTT:
-
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- DMSO:
-
Dimethyl sulfoxide
- GO:
-
Gene Ontology
- PVDF:
-
Polyvinylidene fluoride
References
Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ (1998) Increasing complexity of Ras signaling. Oncogene 17:1395–1413
McCormick F (1989) ras GTPase activating protein: signal transmitter and signal terminator. Cell 56:5–8
Jaffee EM, Hruban RH, Canto M, Kern SE (2002) Focus on pancreas cancer. Cancer Cell 2:25–28
Jemal A, Murray T, Ward E et al (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant cK-ras genes. Cell 53:549–554
Shi X, Liang Z, Ren X, Liu T (2008) Combined silencing of K-ras and Akt2 oncogenes achieves synergistic effects in inhibiting pancreatic cancer cell growth in vitro and in vivo. Cancer Gene Ther 16:227–236
Zhao M, He H, Sun H, Ren K, Shao R (2009) Dual knockdown of N-ras and epiregulin synergistically suppressed the growth of human hepatoma cells. Biochem Biophys Res Commun 387:239–244
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5:275–284
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627
Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA (2005) Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-C directed therapy. Mol Cancer Res 3:413–423
Hopfner M, Schuppan D, Scherubl H (2008) Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol 14:1–14
Moffat J, Grueneberg DA, Yang X et al (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124:1283–1298
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆ Ct method. Methods 25:402–408
Jiang R, Cabras G, Sheng W, Zeng Y, Ooka T (2009) Synergism of BARF1 with Ras induces malignant transformation in primary primate epithelial cells and human nasopharyngeal epithelial cells. Neoplasia 11:964–973
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Ruggero D, Pandolfi PP (2003) Does the ribosome translate cancer? Nat Rev Cancer 3:179–192
Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N (2004) Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2:690–698
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591
DeNicola GM, Karreth FA, Humpton TJ et al (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475:106–109
Fang J, Seki T, Maeda H (2009) Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 61:290–302
Friday BB, Adjei AA (2005) K-ras as a target for cancer therapy. Biochim Biophys Acta 1756:127–144
Ji Z, Mei FC, Lory PL, Gilbertson SR, Chen Y, Cheng X (2009) Chemical genetic screening of KRAS-based synthetic lethal inhibitors for pancreatic cancer. Front Biosci 14:2904–2910
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553
Lee NS, Dohjima T, Bauer G et al (2002) Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 20:500–505
Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P (2006) A versatile tool for conditional gene expression and knockdown. Nat Methods 3:109–116
Brummelkamp TR, Bernards R, Agami R (2002) Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2:243–247
Links M, Brown R (1999) Clinical relevance of the molecular mechanisms of resistance to anti-cancer drugs. Expert Rev Mol Med 1:1–21
Naito S, Yokomizo A, Koga H (1999) Mechanisms of drug resistance in chemotherapy for urogenital carcinoma. Int J Urol 6:427–439
Warner JR, McIntosh KB (2009) How common are extraribosomal functions of ribosomal proteins? Mol Cell 34:3–11
Takagi M, Absalon MJ, McLure KG, Kastan MB (2005) Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123:49–63
Wang Y, Cheong D, Chan S, Hooi SC (1999) Heparin/heparan sulfate interacting protein gene expression is up-regulated in human colorectal carcinoma and correlated with differentiation status and metastasis. Cancer Res 59:2989–2994
Liu JJ, Huang BH, Zhang J, Carson DD, Hooi SC (2006) Repression of HIP/RPL29 expression induces differentiation in colon cancer cells. J Cell Physiol 207:287–292
Liu JJ, Zhang J, Ramanan S, Julian JA, Carson DD, Hooi SC (2004) Heparin/heparan sulfate interacting protein plays a role in apoptosis induced by anticancer drugs. Carcinogenesis 25:873–879
Acknowledgments
We are grateful to Dr Jianzhong Xi (Peking University, Department of Biomedical Engineering College of Engineering, Beijing, China) for lentiviruses production and critical reading of the manuscript. National New Drug Research and Development Project (Grant 2010ZX09401-403) supported this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Ge, M., Yin, Y. et al. Silencing expression of ribosomal protein L26 and L29 by RNA interfering inhibits proliferation of human pancreatic cancer PANC-1 cells. Mol Cell Biochem 370, 127–139 (2012). https://doi.org/10.1007/s11010-012-1404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1404-x