Abstract
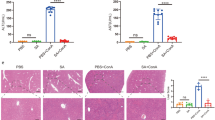
Hepatocellular carcinoma is becoming one of the most prominent types of cancer in the world. Recently, from Styrax japonica Siebold et al. Zuccarini (SJSZ), we isolated a glycoprotein which consists of carbohydrate moiety (52.64%) and protein moiety (42.35%). We evaluated whether SJSZ glycoprotein prevents hepatocarcinogenesis induced by N-nitrosodiethylamine (DEN). The purpose of this study was to evaluate the effect of SJSZ glycoprotein in DEN-induced hepatocarcinogenesis in ICR mice. To know chemopreventive effect of SJSZ glycoprotein on hepatocarcinogenesis, ICR mice were intraperitoneally injected with N-nitrosodiethylamine (DEN, 10 mg/kg) for 7 weeks. After sacrifice, we evaluated indicators of liver tissue damage [the activities of lactate dehydrogenase (LDH) and alanine aminotransferase (ALT), and thiobarbituric acid reactive substances (TBARS)], antioxidative enzymes [activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)], and initiating hepatocarcinogenic indicator [heat shock protein (HSP) 27 and 70] and hepatocarcinogenic signals [protein kinase C (PKC), extracellular signal-regulating kinase (ERK) 1/2, nuclear factor (NF)-κB (p50 and p65) and tumor necrosis factor-α (TNF-α)] using biochemical methods, immunoblot analysis, and RT-PCR. The results obtained from this study revealed that SJSZ glycoprotein (10 mg/kg, BW) decreased the levels of LDH, ALT, and TBARS, whereas the activities of SOD, GPx, and CAT increased in the DEN-induced ICR mice. With respect to the hepatocarcinogenic indicator and hepatocarcinogenic signals, HSP27, HSP70, PKC, ERK1/2, NF-κB (p50 and p65), and TNF-α, activity decreased. Hence, SJSZ glycoprotein might prevent expression of HSP27 and HSP70 by DEN.






Similar content being viewed by others
References
Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, McCombie WR, Wigler M, Hicks J, Hannon GJ, Powers S, Lowe SW (2008) An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell 135:852–864
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121:977–990
Jeena KJ, Joy KL, Kuttan R (1999) Effect of Emblica officinalis, Phyllanthus amarus and Picrorrhiza kurroa on N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Lett 136:11–16
Loeppky RN (1994) Nitrosamine and nitroso compound chemistry and biochemistry, ACS Symposium Series, vol. 553. American Chemical Society, Washington, DC, pp 1–12
Heindryckx F, Colle I, Van Vlierberghe H (2009) Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 90:367–386
Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM (2008) Experimental models of hepatocellular carcinoma. J Hepatol 48:858–879
Nishigori C (2006) Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci 5:208–214
Sundaresan S, Subramanian P (2003) S-allylcysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol 55:37–42
Joo M, Chi JG, Lee H (2005) Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci 20:829–834
Faucher C, Capdevielle J, Canal I, Ferrara P, Mazarguil H, McGuire WL, Darbon JM (1993) The 28-kDa protein whose phosphorylation is induced by protein kinase C activators in MCF-7 cells belongs to the family of low molecular mass heat shock proteins and is the estrogen-regulated 24-kDa protein. J Biol Chem 268:15168–15173
Takai S, Matsushima-Nishiwaki R, Tokuda H, Yasuda E, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O (2007) Protein kinase C delta regulates the phosphorylation of heat shock protein 27 in human hepatocellular carcinoma. Life Sci 81:585–591
Fang JY, Richardson BC (2005) The MAPK signalling pathways and colorectal cancer. Lancet Oncol 6:322–327
Zhang X, Liu S, Hu T, Liu S, He Y, Sun S (2009) Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 50:490–499
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Newman DJ (2008) Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem 51:2589–2599
Kim TJ (1998) Korean resources plants, 1st edn. Seoul National University Press, South Korea
Yoshikawa K, Hirai H, Tanaka M, Arihara S (2000) Antisweet natural products. XV. Structures of Jegosaponins A-D from Styrax japonica Sieb. et Zucc. Chem Pharm Bull 48:1093–1096
Lee J, Lim KT (2011) Inhibitory effect of phytoglycoprotein (38 kDa) on expression of matrix metalloproteinase-9 in 12-O-tetradecanoylphorbol-13-acetate-treated HepG2cells. Naunyn Schmiedebergs Arch Pharmacol 384:185–196
Neville DM Jr, Glossmann H (1974) Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol 32:92–102
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339:69–72
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bergmeyer HU, Bernt E (1974) UV-assay with pyruvate and NADH. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 2. Academic Press, New York, pp 574–578
Bergmeyer HU, Bernt E (1974) Glutamate-pyruvate transaminase: UV assay, manual method. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 2. Academic Press, New York, pp 752–758
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Thomson JF, Nance SL, Tollaksen SL (1978) Spectrophotometric assay of catalase with perborate as substrate. Proc Soc Exp Biol Med 157:33–35
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Hong WK, Sporn MB (1997) Recent advances in chemoprevention of cancer. Science 278:1073–1077
Rohr-Udilova NV, Stolze K, Sagmeister S, Nohl H, Schulte-Hermann R, Grasl-Kraupp B (2008) Lipid hydroperoxides from processed dietary oils enhance growth of hepatocarcinoma cells. Mol Nutr Food Res 52:352–359
Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, Totsuka S (1999) Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer 86:589–595
Vásquez-Garzón VR, Arellanes-Robledo J, García-Román R, Aparicio-Rautista DI, Villa-Treviño S (2009) Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res 43:128–137
Rao GM, Rao CV, Pushpangadan P, Shirwaikar A (2006) Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol 103:484–490
Margaill I, Plotkine M, Lerouet D (2005) Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39:429–443
Sakamoto M (2009) Early HCC: diagnosis and molecular markers. J Gastroenterol 44:108–111
Bishayee A, Waghray A, Barnes KF, Mbimba T, Bhatia D, Chatterjee M, Darvesh AS (2010) Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res 27:1080–1091
Guo K, Liu Y, Zhou H, Dai Z, Zhang J, Sun R, Chen J, Sun Q, Lu W, Kang X, Chen P (2008) Involvement of protein kinase C beta-extracellular signal-regulating kinase 1/2/p38 mitogen-activated protein kinase-heat shock protein 27 activation in hepatocellular carcinoma cell motility and invasion. Cancer Sci 99:486–496
Gollob JA, Wilhelm S, Carter C, Kelley SL (2006) Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol 33:392–406
Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G (2007) Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta 1773:1196–1212
Karin M, Yamamoto Y, Wang QM (2004) The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov 3:17–26
Aggarwal BB, Vijayalekshmi RV, Sung B (2009) Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 15:425–430
Bosch FX, Ribes J, Díaz M, Cléries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–S16
Wu Y, Zhou BP (2010) TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102:639–644
Acknowledgment
This study was financially supported by Chonnam National University, 2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Lim, KT. Activity of tumor necrosis factor-α blocked by phytoglycoprotein (38 kDa) at initiation stage in N-nitrosodiethylamine-induced ICR mice. Mol Cell Biochem 362, 177–186 (2012). https://doi.org/10.1007/s11010-011-1140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1140-7