Abstract
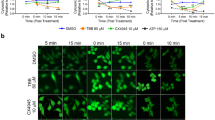
Protein kinase CK2 is implicated in the regulation of the cell cycle. In addition to a variety of functions, CK2 has anti-apoptotic properties. So far the role of CK2 linking both pathways in the cell is not clear. Some years ago we found that CK2 phosphorylates cdc25C, one member of the cdc25 family of proteins. In this study, we showed that inhibition of CK2 activity by three different inhibitors led to a down-regulation of the level of cdc25C. Inhibition of CK2 activity by transfecting the dominant-negative CK2α subunit also resulted in a down-regulation of the level of cdc25C whereas inhibition of CK2α′ had no effect on the cdc25C level. In both cases, we observed apoptosis by PARP cleavage as well as by an increase in γH2AX phosphorylation. These results show that down-regulation of the level of cdc25C is not a prerequisite for the induction of apoptosis.





Similar content being viewed by others
References
Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–291
Nilsson I, Hoffmann I (2000) Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res 4:107–114
Karlsson-Rosenthal C, Millar JB (2006) Cdc25: mechanisms of checkpoint inhibition and recovery. Trends Cell Biol 16:285–292
Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW (1991) cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67:197–211
Perdiguero E, Nebreda AR (2004) Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle 3:733–737
Ouyang B, Li W, Pan H, Meadows J, Hoffmann I, Dai W (1999) The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18:6029–6036
Schwindling SL, Noll A, Montenarh M, Götz C (2004) Mutation of a CK2 phosphorylation site in cdc25C impairs importin α/β binding and results in cytoplasmic retention. Oncogene 23:4155–4165
Litchfield DW, Lüscher B, Lozeman FJ, Eisenman RN, Krebs E (1992) Phosphorylation of CK II by p34cdc2 in vitro and at mitosis. J Biol Chem 267:13943–13951
Mulner-Lorillon O, Cormier P, Labbe J-C, Doree M, Pouhle R, Osborne H, Belle R (1990) M-phase-specific cdc2 protein kinase phosphorylates the β subunit of casein kinase II and increases casein kinase II activity. Eur J Biochem 193:529–534
Bosc DG, Slominski E, Sichler C, Litchfield DW (1995) Phosphorylation of casein kinase II by p34cdc2—identification of phosphorylation sites using phosphorylation site mutants in vitro. J Biol Chem 270:25872–25878
Messenger MM, Saulnier RB, Gilchrist AD, Diamond P, Gorbsky GJ, Litchfield DW (2002) Interactions between protein kinase CK2 and Pin1—evidence for phosphorylation-dependent interactions. J Biol Chem 277:23054–23064
St-Denis NA, Derksen DR, Litchfield DW (2009) Evidence for regulation of mitotic progression through temporal phosphorylation and dephosphorylation of CK2α. Mol Cell Biol 29:2068–2081
Yu IJ, Spector DL, Bae Y-S, Marshak DR (1991) Immunocytochemical localization of casein kinase II during interphase and mitosis. J Cell Biol 114:1217–1232
Faust M, Günther J, Morgenstern E, Montenarh M, Götz C (2002) Specific localization of the catalytic subunits of protein kinase CK2 at the centrosomes. Cell Mol Life Sci 59:2155–2164
Theis-Febvre N, Filhol O, Froment C, Cazales M, Cochet C, Monsarrat B, Ducommun B, Baldin V (2003) Protein kinase CK2 regulates CDC25B phosphatase activity. Oncogene 22:220–232
Ford HL, Landesmann-Bollag E, Dacwag CS, Stukenberg PT, Pardee AB, Seldin DC (2000) Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem 275:22245–22254
Heriche JK, Lebrin F, Rabilloud T, LeRoy D, Chambaz EM, Goldberg Y (1997) Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276:952–955
Escargueil AE, Plisov SY, Filhol O, Cochet C, Larsen AK (2000) Mitotic phosphorylation of DNA topoisomerase II α by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J Biol Chem 275:34710–34718
Schneider CC, Hessenauer A, Montenarh M, Götz C (2010) p53 is dispensable for the induction of apoptosis after inhibition of protein kinase CK2. Prostate 70:126–134
Hessenauer A, Schneider CC, Götz C, Montenarh M (2011) CK2 inhibition induces apoptosis via the ER stress response. Cell Signal 23:145–151
Hessenauer A, Montenarh M, Götz C (2003) Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. Int J Oncol 22:1263–1270
Sinisi AA, Chieffi P, Pasquali D, Kisslinger A, Staibano S, Bellastella A, Tramontano D (2002) EPN: a novel epithelial cell line derived from human prostate tissue. In Vitro Cell Dev Biol Anim 38:165–172
Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P (1995) Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol 31:14–24
Penner CG, Wang ZL, Litchfield DW (1997) Expression and localization of epitope-tagged protein kinase CK2. J Cell Biochem 64:525–537
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–682
Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’). FEBS Lett 496:44–48
St-Denis NA, Litchfield DW (2009) From birth to death: The role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol Life Sci 66:1817–1829
Faust M, Kartarius S, Schwindling SL, Montenarh M (2002) Cyclin H is a new binding partner for protein kinase CK2. Biochem Biophys Res Commun 269:6–12
Schneider E, Montenarh M, Wagner P (1998) Regulation of CAK kinase activity by p53. Oncogene 17:2733–2742
Schneider E, Kartarius S, Schuster N, Montenarh M (2002) The cyclin H/cdk7/Mat1 kinase activity is regulated by CK2 phosphorylation of cyclin H. Oncogene 21:5031–5037
Russo G, Vandenberg M, Yu IY, Bae Y-S, Franza BR, Marshak DR (1992) Casein kinase II phosporylated p34cdc2 kinase in G1 phase of the HeLa cell division cycle. J Biol Chem 267:20317–20325
Li J, Meyer AN, Donoghue DJ (1997) Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc Natl Acad Sci USA 94:502–507
Sadhu K, Reed SI, Richardson H, Russell P (1990) Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc Natl Acad Sci 87:5139–5143
Galaktionov K, Beach D (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell 67:1181–1194
Nagata A, Igarashi M, Jinno S, Suto K, Okayama H (1991) An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol 3:959–968
Kreutzer J, Guerra B (2007) The regulatory beta-subunit of protein kinase CK2 accelerates the degradation of CDC25A phosphatase through the checkpoint kinase Chk1. Int J Oncol 31:1251–1259
Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA (2004) 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun 321:1040–1044
Cozza G, Mazzorana M, Papinutto E, Bain J, Elliott M, Di MG, Gianoncelli A, Pagano MA, Sarno S, Ruzzene M, Battistutta R, Meggio F, Moro S, Zagotto G, Pinna LA (2009) Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem J 421:387–395
Acknowledgments
We thank D. Litchfield, Ontario, for the gift of the original vectors with the CK2 dominant-negative mutants. We also thank Nathaniel Saidu for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carolin C. Schneider and Claudia Götz contributed equally to this study.
Rights and permissions
About this article
Cite this article
Schneider, C.C., Götz, C., Hessenauer, A. et al. Down-regulation of CK2 activity results in a decrease in the level of cdc25C phosphatase in different prostate cancer cell lines. Mol Cell Biochem 356, 177–184 (2011). https://doi.org/10.1007/s11010-011-0946-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0946-7