Abstract
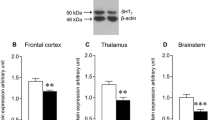
Molecular processes regulating brain stem serotonergic receptors play an important role in the control of respiration. We evaluated 5-HT2A receptor alterations in the brain stem of neonatal rats exposed to hypoxic insult and the effect of glucose, oxygen, and epinephrine resuscitation in ameliorating these alterations. Hypoxic stress increased the total 5-HT and 5-HT2A receptor number along with an up regulation of 5-HT Transporter and 5-HT2A receptor gene in the brain stem of neonates. These serotonergic alterations were reversed by glucose supplementation alone and along with oxygen to hypoxic neonates. The enhanced brain stem 5-HT2A receptors act as a modulator of ventilatory response to hypoxia, which can in turn result in pulmonary vasoconstriction and cognitive dysfunction. The adverse effects of 100% oxygenation and epinephrine administration to hypoxic neonates were also reported. This has immense clinical significance in neonatal care.






Similar content being viewed by others
References
Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A (2000) Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol 475:123–130
Johnston MV, Nakajima W, Hagberg H (2002) Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist 8(3):212–220
Tryba AK, Pena F, Ramirez JM (2006) Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26:2623–2634
Justin EA, Frederic JS, Theodore A (2004) Slotkin developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect 112:148–155
Jackson J, Paulose CS (2000) Brain 5HT2A receptor regulation by tryptophan supplementation in streptozotocin diabetic rats. J Biochem Mole Biol Biophys 5:1–7
Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, Adnot S (1997) Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol 272:H1173–H1181
Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS (2004) BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7(1):48–55
Serrano J, Encinas JM, Salas E, Fernández AP, Castro-Blanco S, Fernández-Vizarra P, Bentura ML, Rodrigo J (2003) Hypobaric hypoxia modifies constitutive nitric oxide synthase activity and protein nitration in the rat cerebellum. Brain Res 976:109–119
Varney AA, Schlenker EH (2007) Thyroid status affects 5-HT2A receptor modulation of breathing before, during, and following exposure of hamsters to acute intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 293(5):R2070–R2080
Abraham PM, Anju TR, Jayanarayanan S, Paulose CS (2010) Serotonergic receptor functional up regulation in cerebral cortex and down regulation in brain stem of Streptozotocin induced diabetic rats: antagonism by pyridoxine and insulin. Neurosci Lett 483:23–27
Reinebrant HE, Wixey JA, Gobe GC, Colditz PB, Buller KM (2010) Differential effects of neonatal hypoxic-ischemic brain injury on brainstem serotonergic raphe nuclei. Brain Res 1322:124–133
Richter DW, Manzke T, Wilken B, Ponimaskin E (2003) Serotonin receptors: guardians of stable breathing. Trends Mol Med 9:542–548
Launay JM, Callebert J, Bondoux D, Loric S, Maroteaux L (1994) Serotonin receptors and therapeutics. Cell Mol Biol 40(3):327–336
Miller KJ, Hoffman BJ (1994) Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J Biol Chem 269(44):27351–27356
Yura A, Kiuchi Y, Uchikawa T, Uchida J, Yamazaki K, Oguchi K (1996) Possible involvement of calmodulin-dependent kinases in Ca(2+)-dependent enhancement of [3H]5-hydroxytryptamine uptake in rat cortex. Brain Res 738(1):96–102
Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S (2000) Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 105:1555–1562
Paul SA, Simons JW, Mabjeesh NJ (2004) HIF at the crossroads between ischemia and carcinogenesis. J Cell Physiol 200:20–30
Wang GB, Wang XQ, Luo GX, He WF, Chen XW, Wu J, Bian XW (2007) Design of a multifunction medical experiment platform and its temperature regulation in cell in vitro culture. J Third Mil Med Univ 29:1289–1292
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain: the disposition of [3H] Norepinephrine, [3H] DOPA in various regions of the brain. J Neurochem 13:655–669
Uzbekov MN, Murphy S, Rose SPR (1979) Ontogenesis of serotonin ‘receptors’ in different regions of rat brain. Brain Res 168:195–199
Leysen JE, Neimegeers CJE, Van Nueten JM, Laduron PM (1982) [3H] Ketanserin, a selective ligand for serotonin2 receptor binding sites. Mol Pharmacol 21:301–314
Lowry OH, Rosenbrough NH, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Scatchard G (1949) The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 51:660–672
Tagliaferro P, Ramos AJ, López-Costa JJ, López EM, Saavedra JP, Brusco A (2001) Increased nitric oxide synthase activity in a model of serotonin depletion. Brain Res Bull 54:199–205
Henley WN, Bellush LL, Notestine MA (1992) Hypoxic attenuation of brain stem serotonin does not influence sodium-induced hypertension. Clin Exp Hypertens A 14:413–433
Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM (1999) Neurotransmitters and neuromodulators controlling the hypoxic respiratory responses in anaesthesized cats. J Physiol 514:567–578
Taylor AH, Nattie EE (2005) Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566:543–557
Pena F, Ramirez JM (2002) Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22:11055–11064
Davis JN, Carlsson A (1973) Effect of hypoxia on tyrosine and tryptophan hydroxylation in unanaesthetized rat brain. J Neurochem 20:913–915
Poncet L, Denoroy L, Dalmaz Y, Pequignot JM (1997) Activity of tryptophan hydroxylase and content of indolamines in discrete brain regions after a long-term hypoxic exposure in the rat. Brain Res 765:122–128
Steiner AA, Branco LGS (2003) Fever and anapyrexia in systemic inflammation: intracellular signaling by cyclic nucleotides. Front Biosci 8:1398–1408
Gargaglioni LH, Steiner AA, Branco LGS (2005) Involvement of serotoninergic receptors in the anteroventral preoptic region on hypoxia-induced hypothermia. Brain Res 1044:16–24
Shartau RB, Tam R, Patrick S, Goldberg JI (2010) Serotonin prolongs survival of encapsulated pond snail embryos exposed to long-term anoxia. J Exp Biol 213:1529–1535
Yamada J, Sugimoto Y, Horisaka K (1995) Serotonin2 (5-HT2) receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) inhibits chlorpromazine- and haloperidol-induced hypothermia in mice. Biol Pharm Bull 18(11):1580–1583
Hommer D, Andreasen P, Rio D, Williams W, Ruttimann U, Momenan R, Zametkin A, Rawlings R, Linnoila M (1997) Effects of m-chlorophenylpiperazine on regional brain glucose utilization: a positron emission tomographic comparison of alcoholic and control subjects. J Neurosci 17(8):2796–2806
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Li Y, Powers C, Jiang N, Chopp M (1998) Intact, injured, necrotic, and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci 156:119–132
Wrona MZ, Dryhurst G (1991) Interactions of 5-hydroxytryptamine with oxidative enzymes. Biochem Pharmacol 41:1145–1162
Ikeda Y, Anderson JH, Long DM (1989) Oxygen free radicals in the genesis of traumatic and peritumoral brain edema. Neurosyrgery 24:679–685
Sakamoto A, Ohnishi ST, Ohnishi T, Ogawa R (1991) Relationship between free radical production and lipid peroxidation during ischemia-reperfusion injury in the rat brain. Brain Res 554:186–192
Karacaoglu E, Bayram I, Celiköz B, Zienowicz RJ (2007) Does sustained epinephrine release trigger a hypoxia-neovascularization cascade. Plast Reconstr Surg 119:858–864
Jensen A, Künzel W, Kastendieck E (2009) Epinephrine and norepinephrine release in the fetus after repeated hypoxia. J Perinat Med 10:109–110
Shah BH, Siddiqui A, Qureshi KA, Khan M, Rafi S, Ujan VA, Yakoob MY, Rasheed H, Saeed SA (1999) Co-activation of Gi and Gq proteins exerts synergistic effect on human platelet. Exp Mol Med 31:42–46
Semenza GL (1999) Perspectives on oxygen sensing. Cell 98:281–284
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97:9082–9087
Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL (2001) HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61:6669–6673
Li Y, Zhou C, Calvert JW, Colohan AR, Zhang JH (2005) Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol 191:198–210
Halterman MW, Federoff HJ (1999) HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol 159:65–72
Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR (2005) Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem 280:3996–4003
Vangeison G, Carr D, Federoff HJ, Rempe DA (2008) The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci 28:1988–1993
Del Duca D, Wong G, Trieu P, Rodaros D, Kouremenos A, Tadevosyan A, Vaniotis G, Villeneuve LR, Tchervenkov CI, Nattel S, Allen BG, Hébert TE, Rohlicek CV (2009) Association of neonatal hypoxia with lasting changes in left ventricular gene expression: an animal model. J Thorac Cardiovasc Surg 138(3):538–546
Acknowledgments
This study was supported by the research grants from DBT, DST, ICMR, Govt. of India and KSCSTE, Govt. of Kerala to Dr. C. S. Paulose. Anju T R thanks Council of Scientific and Industrial Research for Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anju, T.R., Korah, P.K., Jayanarayanan, S. et al. Enhanced brain stem 5HT2A receptor function under neonatal hypoxic insult: role of glucose, oxygen, and epinephrine resuscitation. Mol Cell Biochem 354, 151–160 (2011). https://doi.org/10.1007/s11010-011-0814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0814-5