Abstract
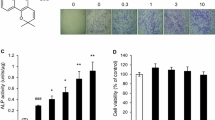
There is an urgent need to develop methods that lower costs of using recombinant human bone morphogenetic proteins (BMPs) to promote bone induction. In this study, we demonstrate the osteogenic effect of a low-molecular weight compound, SVAK-12, that potentiated the effects of BMP-2 in inducing transdifferentiation of C2C12 myoblasts into the osteoblastic phenotype. Here, we report a specific compound, SVAK-12, which was selected based on in silico screenings of small-molecule databases using the homology modeled interaction motif of Smurf1-WW2 domain. The enhancement of BMP-2 activity by SVAK-12 was characterized by evaluating a BMP-specific reporter activity and by monitoring the BMP-2-induced expression of mRNA for osteocalcin and alkaline phosphatase (ALP), which are widely accepted marker genes of osteoblast differentiation. Finally, we confirmed these results by also measuring the enhancement of BMP-2-induced activity of ALP. Smurf1 is an E3 ligase that targets osteogenic Smads for ubiquitin-mediated proteasomal degradation. Smurf1 is an interesting potential target to enhance bone formation based on the positive effects on bone of proteins that block Smurf1-binding to Smad targets or in Smurf1−/− knockout mice. Since Smads bind Smurf1 via its WW2 domain, we performed in silico screening to identify compounds that might interact with the Smurf1-WW2 domain. We recently reported the activity of a compound, SVAK-3. However, SVAK-3, while exhibiting BMP-potentiating activity, was not stable and thus warranted a new search for a more stable and efficacious compound among a selected group of candidates. In addition to being more stable, SVAK-12 exhibited a dose-dependent activity in inducing osteoblastic differentiation of myoblastic C2C12 cells even when multiple markers of the osteoblastic phenotype were parallelly monitored.









Similar content being viewed by others
Abbreviations
- BMP:
-
Bone morphogenetic protein
- Smurf1:
-
Smad ubiquitin regulatory factor-1
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- ALP:
-
Alkaline phosphatase
- DMSO:
-
Dimethylsulfoxide
- RUL:
-
Relative units of luciferase
- FBS:
-
Fetal bovine serum
- CAP database:
-
Chemicals available for purchase database
References
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22:233–241
Polly DW Jr, Ackerman SJ, Shaffrey CI, Ogilvie JW, Wang JC, Stralka SW, Mafilios MS, Heim SE, Sandhu HS (2003) A cost analysis of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. Orthopedics 26:1027–1037
Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27:2662–2673
Boden SD, Schimandle JH, Hutton WC (1995) The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine 20:2633–2644
Kusumoto K, Bessho K, Fujimura K, Akioka J, Okubo Y, Wang Y, Iizuka T, Ogawa Y (2002) Osteoinduction by recombinant human bone morphogenetic protein-2 in muscles of non-human primates. J Int Med Res 30:251–259
Ackerman SJ, Mafilios MS, Polly DW Jr (2002) Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion: an evidence-based modeling approach. Spine 27:S94–S99
Yamashita M, Ying S, Zhang G, Li C, Cheng SY, Deng C, Zhang Y (2005) Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 121:101–113
Zhao M, Qiao M, Harris SE, Oyajobi BO, Mundy GR, Chen D (2004) Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. J Biol Chem 279:12854–12859
Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L (2006) LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem 281:17212–17219
Sangadala S, Okada M, Liu Y, Viggeswarapu M, Titus L, Boden SD (2009) Engineering, cloning and functional characterization of recombinant LIM mineralization protein-1 containing an N-terminal HIV-derived membrane transduction domain. Protein Expr Purif 65:165–173
Sangadala S, Boden SD, Metpally RP, Reddy BV (2007) Modeling and analysis of molecular interaction between Smurf1-WW2 domain and various isoforms of LIM mineralization protein. Proteins 68:690–701
Bohm HJ (1992) The computer program LUDI: a new method for the de novo design of enzyme inhibitors. J Comput Aided Mol Des 6:61–78
Sangadala S, Metpally RP, Reddy BV (2007) Molecular interaction between Smurf1 WW2 domain and PPXY motifs of Smad1, Smad5, and Smad6—modeling and analysis. J Biomol Struct Dyn 25:11–23
Bohm HJ (1994) On the use of LUDI to search the fine chemicals directory for ligands of proteins of known three-dimensional structure. J Comput Aided Mol Des 8:623–632
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Okada M, Sangadala S, Liu Y, Yoshida M, Reddy BV, Titus L, Boden SD (2009) Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity. Cell Biochem Funct 27:526–534
Disclosures
All the biochemical studies in this work were performed at the Atlanta Veterans Affairs Medical Center and partly supported by NIH grant # R01 AR53093 (Boden) and a VA Merit award to Dr. Titus. In the past and not related to this work, Dr. Boden has received compensation as a consultant for Medtronic Sofamor Danek and for intellectual property. Emory University and some of the authors have/may receive royalties in the future related to LMP-1. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. We thank Boojala VB Reddy for assistance in homology modeling. We also thank Vandana Voleti for assistance in computational analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Satoshi Kato and Sreedhara Sangadala contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kato, S., Sangadala, S., Tomita, K. et al. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Mol Cell Biochem 349, 97–106 (2011). https://doi.org/10.1007/s11010-010-0664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0664-6