Abstract
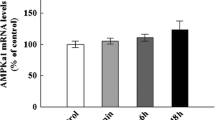
Phenylketonuria (PKU) is characterized by elevated levels of phenylalanine (Phe) in plasma and cerebrospinal fluid of PKU patients, leading to mental retardation. The developmental delay in the cerebral cortex is one of the characteristic pathologic changes in untreated phenylketonuria patients. This is thought to be due to the toxic effects of Phe and/or its metabolites; however, the underlying mechanisms are as yet unknown. In this study, using a model system in which cultured cortical neurons were induced with Phe, we observed that Phe inhibited the longest neurite outgrowth and induced the neuronal death. We further demonstrated that the expression of BDNF mRNA and protein was significantly decreased by Phe, together with a decrease in extracellular signal-regulated kinase (ERK) and Akt phosphorylation activity. There was no change in expression of TrkB mRNA and protein. Considering the important role of BDNF in normal brain development and function, these l-Phe-induced changes in BDNF in PKU brain may be a critical element of the neurological symptoms of PKU.




Similar content being viewed by others
References
Kienzle Hagen ME, Pederzolli CD, Sgaravatti AM et al (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586(3):344–352
Horster F, Schwab MA, Sauer SW et al (2006) Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res 59(4):544–548
Pietz J, Rupp A, Ebinger F et al (2003) Cerebral energy metabolism in phenylketonuria: findings by quantitative in vivo 31P MR spectroscopy. Pediatr Res 53:654–662
Pascucci T, Ventura R, Puglisi-Allegra S et al (2002) Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport 13:2561–2564
Shefer S, Tint GS, Jean-Guillaume D et al (2000) Is there a relationship between 3-hydroxy-3-methylglutaryl coenzyme a reductase activity and forebrain pathology in the PKU mouse? J Neurosci Res 61(5):549–563
Dyer CA, Kendler A, Philibotte T et al (1996) Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol 55(7):795–814
Bauman ML, Kemper TL (1982) Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol 58(1):55–63
Martynyuk AE, Glushakov AV, Sumners C et al (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol Genet Metab 86(S1):S34–S42
Glushakov AV, Glushakova O, Varshney M et al (2005) Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain 128(Pt 2):300–307
Glushakov AV, Dennis DM, Sumners C et al (2003) L-phenylalanine selectively depresses currents at glutamatergic excitatory synapses. J Neurosci Res 72(1):116–124
Huttenlocher PR (2000) The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159(S2):s102–s106
Zhang Y, Zhang H, Yuan X et al (2007) Differential effects of phenylalanine on Rac1, Cdc42, and RhoA expression and activity in cultured cortical neurons. Pediatr Res 62(1):8–13
Hartwig C, Gala A, Santerb R et al (2006) Elevated phenylalanine levels interfere with neurite outgrowth stimulated by the neuronal cell adhesion molecule L1 in vitro. FEBS Lett 580:3489–3492
Lu B, Chow A (1999) Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res 58:76–87
Schinder AF, Poo M (2000) The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci 23:639–645
Encinas M, Iglesias M, Llecha N et al (1999) Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem 73(4):1409–1421
Almeida RD, Manadas BJ, Melo CV et al (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12(10):1329–1343
Micheli MR, Bova R, Laurenzi MA et al (2006) Modulation of BDNF and TrkB expression in rat hippocampus in response to acute neurotoxicity by diethyldithiocarbamate. Neurosci Lett 410(1):66–70
Ge Y, Belcher SM, Light KE (2004) Alterations of cerebellar mRNA specific for BDNF, p75NTR, and TrkB receptor isoforms occur within hours of ethanol administration to 4-day-old rat pups. Brain Res Dev Brain Res 151(1–2):99–109
Tapia-Arancibia L, Aliaga E, Silhol M et al (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59(1):201–220
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5(6):311–322
Ferrer I, Goutan E, Marín C et al (2000) Brain-derived neurotrophic factor in Huntington disease. Brain Res 866(1–2):257–261
Zhang H, Gu XF (2005) A study of gene expression profiles of cultured embryonic rat neurons induced by phenylalanine. Metab Brain Dis 20:61–72
Strittmatter SM, Fishman MC, Zhu X-P (1994) Activated mutants of the alpha subunit of G(o) promote an increased number of neurites per cell. J Neurosci 14(4):2327–2338
Kornguth S, Gilbert-Barness E, Langer E et al (1992) Golgi-Kopsch silver study of the brain of a patient with untreated phenylketonuria, seizures and cortical blindness. Am J Med Gen 44:443–448
Cordero ME, Trejo M, Colombo M et al (1983) Histological maturation of the neocortex in phenylketonuric rats. Early Hum Dev 8:157–173
Gu XF, Yang XW, Chen RG (2000) Possible mechanism of nerve damage on hyperphenylalanine in embryonic rat. Am J Hum Genet 67:S281
Yang XW, Gu XF, Chen RG (2000) Toxic effects of phenylacetic acid to cultured rat cortical neurons. Chinese J Neurosci 16:330–332
Hannigan JH, Saunders DE, Treas LM (1999) Modification of alcohol-related neurodevelopmental disprders: in vitro and in vivo studies of neuroplasticity. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR et al (eds) Alcohol and alcoholism. Lawrence Erlbaum Associates, Mahwah, New Jersey, pp 39–58
Sapolsky RM, Uno H, Rebert CS et al (1990) Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10:2897–2902
Lapchak PA, Hefti F (1992) BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. NeuroReport 3:405–408
Middlemas DS, Lindberg RA, Hunter T (1991) TrkB, a neural receptor protein-tyrosine kinase: evidence for a fulllength and two truncated receptors. Mol Cell Biol 11:143–153
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Soto I, Rosenthal JJC, Blagburn JM (2006) Fibroblast growth factor 2 applied to the optic nerve after axotomy up-regulates BDNF and TrkB in ganglion cells by activating the ERK and PKA signaling pathways. J Neurochem 96:82–96
Davis MI (2008) Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther 118(1):36–57
McAllister AK, Lo DC, Katz LC (1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15(4):791–803
McAllister AK, Katz LC, Lo DC (1997) Opposing roles for endogenous BDNF and NT-3 in regulating cotical dendritic growth. Neuron 18:767–778
Horsh HW, Knuttgen A, Portbury SD et al (1999) Destabilization of cortical dendrites and spines by BDNF. Neuron 23:353–364
Brunet A, Bonni A, Zigmond MJ et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868
Duarte AI, Santos P, Oliveira CR et al (2008) Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta 1783(6):994–1002
Allan LA, Morrice N, Brady S et al (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5(7):647–654
Kao S, Jaiswal RK, Kolch W et al (2001) Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem 276:18169–18177
Schmitt JM, Wayman GA, Nozaki N et al (2004) Calcium activation of ERK mediated by calmodulin kinase I. J Biol Chem 279(23):24064–24072
Yu YG, Tang FG, Pan J et al (2007) Effects of phenylalanine and its metabolites on cytoplasmic free calcium in cortical neurons. Neurochem Res 32(8):1292–1301
Acknowledgments
This work was supported by Hi-Tech Research and Development Program (2007AA02Z447), and Shanghai government foundations (Z07ZZ33, 2008ZD001, 08JC1416100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Gu, X., Lu, L. et al. Effects of phenylalanine on the survival and neurite outgrowth of rat cortical neurons in primary cultures: possible involvement of brain-derived neurotrophic factor. Mol Cell Biochem 339, 1–7 (2010). https://doi.org/10.1007/s11010-009-0364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0364-2