Abstract
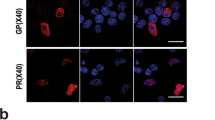
Sandhoff disease (SD) is a lysosomal storage disorder due to mutations in the gene encoding for the β-subunit of β-hexosaminidase, that result in β-hexosaminidase A (αβ) and β-hexosaminidase B (ββ) deficiency. This leads to the storage of GM2 ganglioside in endosomes and lysosomes, which ends in a progressive neurodegeneration. Currently, very little is known about the biochemical pathways leading from GM2 ganglioside accumulation to pathogenesis. Defects in transport and sorting by the endosomal–lysosomal system have been described for several lysosomal storage disorders. Here, we have investigated the endosomal–lysosomal compartment in fibroblasts from SD patients and observed that both late endosomes and lysosomes, but not early endosomes, have a higher density in comparison with normal fibroblasts. Moreover, Sandhoff fibroblasts have an intracellular distribution of terminal endocytic organelles that differs from the characteristic perinuclear punctate pattern observed in normal fibroblasts and endocytic vesicles also appear larger. These findings reveal the occurrence of an alteration in the terminal endocytic organelles of Sandhoff fibroblasts, suggesting an involvement of this compartment in the disruption of cell metabolic and signalling pathways and in the onset of the pathological state.








Similar content being viewed by others
References
Stoscheck CM, Carpenter G (1984) Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol 98:1048–1053
Trejo J, Hammes SR, Coughlin SR (1998) Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci USA 95:3698–3702
Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3:122–131
Reddy A, Caler EV, Andrews NW (2001) Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106:157–169
Cataldo AM, Peterhoff CM, Troncoso JC et al (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 157:277–286
Scriver CR, Beaudet AL, Sly WS, Valle DD (eds) (2001) Lysosomal disorders. The metabolic and molecular bases of inherited disease, 8th edn, vol III. McGraw-Hill, New York, pp 3371–3894
Jeyakumar M, Dwek RA, Butters TD, Platt FM (2005) Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci 6:713–725
Ginzburg L, Kacher Y, Futerman AH (2004) The pathogenesis of glycosphingolipid storage disorder. Semin Cell Dev Bio 15:417–431
Bahr BA, Bendiske J (2002) The neuropathogenic contributions of lysosomal dysfunction. J Neurochem 83:481–489
Ivleva TS, Ogloblina TA, Litinskaya LL, Wiederschain GY (1991) Estimation and comparison of lysosomal and cytoplasmic pH of human fibroblasts from healthy donors and patients with lysosomal storage diseases. Biomed Sci 2:398–402
Bach G, Chen CS, Pagano RE (1999) Elevated lysosomal pH in Mucolipidosis type IV cells. Clin Chim Acta 280:173–179
Schmid JA, Mach L, Paschke E, Glössl J (1999) Accumulation of sialic acid in endocytic compartments interferes with the formation of mature lysosomes. J Biol Chem 274:19063–19071
Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y et al (2006) TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem 281:7294–7301
Pagano RE (2003) Endocytic trafficking of glycosphingolipids in sphingolipid storage diseases. Philos Trans R Soc Lond B Biol Sci 358:885–891 Review
Sillence DJ, Platt FM (2004) Glycosphingolipids in endocytic membrane transport. Semin Cell Dev Biol 15:409–416
Vruchte D, Lloyd-Evans E, Vldman RJ et al (2004) Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J Biol Chem 279:26167–26175
Martino S, Emiliani C, Tancini B et al (2002) Absence of metabolic cross-correction in Tay-Sachs cells: implications for gene therapy. J Biol Chem 277:20177–20184
Mahuran DJ (1999) Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim Biophys Acta 1455:105–138
Mencarelli S, Cavalieri C, Magini A et al (2005) Identification of plasma membrane associated β-Hexosaminidase A, active towards GM2 ganglioside, in human fibroblasts. FEBS Lett 579:5501–5506
Magini A, Mencarelli S, Tancini B et al (2008) Identification and characterization of mature beta-hexosaminidases associated with human placenta lysosomal membrane. Biosci Rep 28:229–237
Bifsha P, Landry K, Ashmarina L et al (2007) Altered gene expression in cells from patients with lysosomal storage disorders suggests impairment of the ubiquitin pathway. Cell Death Differ 14:511–523
Connolly GP (1998) Fibroblast models of neurological disorders: fluorescence measurement studies. Trends Pharmacol Sci 19:171–177
Zampieri S, Filocamo M, Buratti E et al (2009) Molecular and functional analysis of the HEXB gene in Italian patients affected with Sandhoff disease: identification of six novel alleles. Neurogenetics 10:49–58
Yeyeodu S, Ahn K, Madden V et al (2000) Procathepsin L self-association as a mechanism for selective secretion. Traffic 1:724–737
Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685
Emiliani C, Beccari T, Tabilio A et al (1990) An enzyme with properties similar to those of beta N-acetylhexosaminidase S is expressed in the promyelocytic cell line HL-60. Biochem J 267:111–117
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75:3327–3331
Keesey J (ed) (1987) Biochemica information, 1st edn. Boehringer Mannheim Biochemicals, Indianapolis, pp 19–20
Putter J, Becker R (1983) Peroxidase. In: Bergmeyer HW (ed) Methods of enzymatic analysis, vol III, 3rd edn. Verlag-Chemie, Weinheim, Germany, pp 286–293
Martinez O, Goud B (1998) Rab proteins. Biochim Biophys Acta 1404:101–112 Review
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Eskelinen EL, Tanaka Y, Saftig P (2003) At the acidic edge: emerging function for lysosomal membrane proteins. Trends Cell Biol 13:137–145
D’Azzo A, Hoogeveen A, Reuser AJ, Robinson D, Galjaard H (1982) Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci USA 79:4535–4539
Mu F-T (1995) EEA1, an early endosome-associated protein. J Biol Chem 270:13503–13511
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480
Rosenfeld JL, Moore RH, Zimmer KP et al (2001) Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J Cell Sci 114:4499–4508
Lebrand C, Corti M, Goodson H et al (2002) Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J 21:1289–1300
Acknowledgments
This work was supported by COFIN-PRIN (Cofinanziamento-Progetto di Ricerca di Interesse Nazionale) and FIRB (Fondo per gli Investimenti della Ricerca di Base) grants to C.E. This work was also supported by Fondazione Cassa di Risparmio di Perugia, Grant 2008.021.375 to C.E. We thank the “Diagnosi PrePostnatale Malattie Metaboliche” Laboratory (G.Gaslini Institute) for providing us with specimens from the “Cell line and DNA bank from patients affected by Genetic diseases” Biobank- Telethon Genetic Biobank Network (project no. GTB07001A). We thank Dr. Maria Ragano Caracciolo for the valuable comments and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tancini, B., Magini, A., Latterini, L. et al. Occurrence of an anomalous endocytic compartment in fibroblasts from Sandhoff disease patients. Mol Cell Biochem 335, 273–282 (2010). https://doi.org/10.1007/s11010-009-0277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0277-0