Abstract
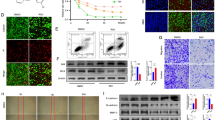
This study first investigates the anti-metastatic effect of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MMPs and u-PA expressions in human lung cancer cells, A549. First, the result demonstrated plumbagin could inhibit TPA induced the abilities of the adhesion, invasion, and migration by cell–matrix adhesion assay and Boyden chamber assay. Data also showed plumbagin could inhibit the activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) involved in the down-regulating enzyme activities, protein and messenger RNA levels of matrix metalloproteinase-2 (MMP-2), and urokinase-type plasminogen activator (u-PA) induced by TPA. Next, plumbagin also strongly inhibited TPA-induced phosphorylation and degradation of inhibitor of kappaBα (IκBα), and the nuclear levels of nuclear factor kappa B (NF-κB), c-Fos, and c-Jun. Also, a dose-dependent inhibition on the binding abilities of NF-κB and activator protein-1 (AP-1) by plumbagin treatment was further observed. Further, the treatment of specific inhibitor for ERK (U0126) to A549 cells could inhibit TPA-induced MMP-2 and u-PA expressions along with an inhibition on cell invasion and migration. Presented data reveals that plumbagin is a novel, effective, anti-metastatic agent that functions by down-regulating MMP-2 and u-PA gene expressions.







Similar content being viewed by others
Abbreviations
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- MMPs:
-
Matrix metalloproteinases
- u-PA:
-
Urokinase-type plasminogen activator
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular signaling-regulating kinase
- JNK/SAPK:
-
c-Jun N-terminal kinase/stress-activated protein kinase
- p38 MAPK:
-
p38 Mitogen-activated protein kinase
- PI3K/Akt:
-
Phosphoinositide 3-kinase/protein kinase B
- NF-κB:
-
Nuclear factor kappa B
- AP-1:
-
Activator protein-1
- IκB:
-
Inhibitor of NF-κB
- IκK:
-
IκB kinase
References
Mossa JS, El-Feraly FS, Muhammad I (2004) Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother Res 18:934–937
Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G (2004) Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog 40:201–211
Ding Y, Chen ZJ, Liu S, Che D, Vetter M, Chang CH (2005) Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J Pharm Pharmacol 57:111–116
Hsieh YJ, Lin LC, Tsai TH (2005) Determination and identification of plumbagin from the roots of Plumbago zeylanica L. by liquid chromatography with tandem mass spectrometry. J Chromatogr A 1083:141–145
Chan-Bacab MJ, Pena-Rodriguez LM (2001) Plant natural products with leishmanicidal activity. Nat Prod Rep 18:674–688
Krishnaswamy M, Purushothaman KK (1980) Plumbagin: a study of its anticancer, antibacterial & antifungal properties. Indian J Exp Biol 18:876–877
Sharma I, Gusain D, Dixit VP (1991) Hypolipidaemic and antiatherosclerotic effects of plumbagin in rabbits. Indian J Physiol Pharmacol 35:10–14
Acharya BR, Bhattacharyya B, Chakrabarti G (2008) The natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin binding. Biochemistry 47:7838–7845
Powolny AA, Singh SV (2008) Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res 25:2171–2180
Shih YW, Lee YC, Wu PF, Lee YB, Chiang T-A (2009) Plumbagin inhibits invasion and migration of liver cancer HepG2 cells by decreasing productions of matrix metalloproteinase-2 and urokinase-plasminogen activator. Hepatol Res. Accepted 12 May 2009
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics. CA Cancer J Clin 51:15–36
Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF (2003) Apoptosis and lung cancer: a review. J Cell Biochem 88:885–898
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Huang Q, Shen HM, Ong CN (2004) Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol 68:361–371
Huang SC, Ho CT, Lin-Shiau SY, Lin JK (2005) Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol 69:221–232
Bernhard EJ, Gruber SB, Muschel RJ (1994) Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci USA 91:4293–4297
Duffy MJ, Duggan C (2004) The urokinase plasminogen activator system: a rich source of tumour markers for the individualized management of patients with cancer. Clin Biochem 37:541–548
Itoh Y, Nagase H (2002) Matrix metalloproteinases in cancer. Essays Biochem 38:21–36
Jang BC, Park YK, Choi IH, Kim SP, Hwang JB, Baek WK, Suh MH, Mun KC, Suh SI (2007) 12-O-tetradecanoyl phorbol 13-acetate induces the expression of B7-DC, -H1, -H2, and -H3 in K562 cells. Int J Oncol 31:1439–1447
Ibañez-Tallon I, Caretti G, Blasi F, Crippa MP (1999) In vivo analysis of the state of the human u-PA enhancer following stimulation by TPA. Oncogene 18:2836–2845
Carpenter CL, Cantley LC (1996) Phosphoinositide kinases. Curr Opin Cell Biol 8:153–158
Chung TW, Lee YC, Kim CH (2004) Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J 18:1123–1125
Chen PN, Hsieh YS, Chiou HL, Chu SC (2005) Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact 156:141–150
Kwon GT, Cho HJ, Chung WY, Park KK, Moon A, Park JH (2008) Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: possible mediation by decreased JNK/AP-1 signaling. J Nutr Biochem Sep 26 (In print)
Lee SJ, Park SS, Lee US, Kim WJ, Moon SK (2008) Signaling pathway for TNF-alpha-induced MMP-9 expression: Mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int Immunopharmacol 8:1821–1826
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Sliva D (2004) Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets 4:327–336
Westermarck J, Kahari VM (1999) Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13:781–792
Aguirre Ghiso JA, Alonso DF, Farias EF, Gomez DE, de Kier Joffe EB (1999) Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur J Biochem 263:295–304
Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW (1995) Coupling of a signal response domain in IκBa to multiple pathways for NF-kB activation. Mol Cell Biol 15:2809–2818
Hoppe-Seyler F, Butz K, Rittmuller C, von Knebel Doeberitz M (1991) A rapid microscale procedure for the simultaneous preparation of cytoplasmic RNA, nuclear DNA binding proteins and enzymatically active luciferase extracts. Nucleic Acids Res 19:5080
Erridge SC, Moller H, Price A, Brewster D (2007) International comparisons of survival from lung cancer: pitfalls and warnings. Nat Clin Pract Oncol 4:570–577
Gajra A, Newman N, Gamble GP, Abraham NZ, Kohman LJ, Graziano SL (2003) Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer 42:51–57
McCracken M, Olsen M, Chen MS Jr, Jemal A, Thun M, Cokkinides V, Deapen D, Ward E (2007) Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 57:190–205
Auerbach O, Garfinkel L, Parks VR (1975) Histologic types of lung cancer in relation to smoking habits, year of diagnosis and sites of metastasis. Chest 67:382–387
Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R (2006) Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst 98:846–855
Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 15:1953–1962
Stetler-Stevenson WG, Aznavoorian S, Liotta LA (1993) Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9:541–573
Kleiner DE, Stetler-Stevenson WG (1999) Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 43:S42–S51
Nee L, Tuite N, Ryan MP, McMorrow T (2007) TNF-alpha and IL-1 beta-mediated regulation of MMP-9 and TIMP-1 in human glomerular mesangial cells. Nephron Exp Nephrol 107:e73–e86
Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A (2007) Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci 48:4360–4367
Acknowledgment
This work was supported by the grant from the Subsidized Project of the Chung Hwa University, Tainan, Taiwan (97-HT-08011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shieh, JM., Chiang, TA., Chang, WT. et al. Plumbagin inhibits TPA-induced MMP-2 and u-PA expressions by reducing binding activities of NF-κB and AP-1 via ERK signaling pathway in A549 human lung cancer cells. Mol Cell Biochem 335, 181–193 (2010). https://doi.org/10.1007/s11010-009-0254-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0254-7