Abstract
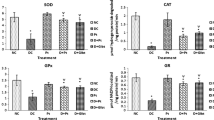
The objective of this study was to verify the effect of the organochalcogen 3-butyl-1-phenyl-2-(phenyltelluro)oct-en-1-one on some parameters of oxidative stress in human serum. Serum of volunteers were incubated for 30 min in the presence or absence of 1, 10, or 30 μM of 3-butyl-1-phenyl-2-(phenyltelluro)oct-en-1-one and oxidative stress was measured. First, we tested the influence of the compound on 1,1-diphenyl-2-picrylhydrazyl (DPPH•) radical-scavenging and verified that the organotellurium did not have any antioxidant properties. The organochalcogen was capable to enhance TBARS but the compound was not able to alter carbonyl assay. Furthermore, the organochalcogen provoked a reduction of protein thiol groups measured by the sulfhydryl assay. Moreover, the organotellurium enhanced the activity of catalase and superoxide dismutase, inhibited the activity of glutathione peroxidase and did not modify the glutathione S-transferase activity. Furthermore, nitric oxide production and hydroxyl radical activity were not affected by the compound. Our findings showed that this organochalcogen induces oxidative stress in human serum, indicating that this compound is potentially toxic to human beings.



Similar content being viewed by others
References
Yarema MC, Curry SC (2005) Acute tellurium toxicity from ingestion of metal-oxidizing solutions. Pediatrics 116:319–321
Yamada N, Kojima R, Uno M, Akiyama T, Kitaura H, Narumi K, Nishiuchi K (2002) Phase-change material for use in rewritable dual-layer optical disk. SPIE 4342:4355–4363
Meotti FC, Borges VC, Zeni G, Rocha JB, Nogueira CW (2003) Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and Ebselen for rats and mice. Toxicol Lett 143:9–16
Savegnago L, Borges VC, Alves D, Jesse CR, Rocha JB, Nogueira CW (2006) Evaluation of antioxidant activity and potential toxicity of 1-buthyltelurenyl-2-methylthioheptene. Life Sci 79:1546–1552
Rooseboom M, Vermeulen NP, Durgut F, Commandeur JN (2002) Comparative study on the bioactivation mechanisms and cytotoxicity of Te-phenyl-l-tellurocysteine, Se-phenyl-l-selenocysteine, and S-phenyl-l-cysteine. Chem Res Toxicol 15:1610–1618
Sailer BL, Liles N, Dickerson S, Chasteen TG (2003) Cytometric determination of novel organotellurium compound toxicity in a promyelocitic (HL-60) cell line. Arch Toxicol 77:30–36
Sailer BL, Liles N, Dickerson S, Sumners S, Chasteen TG (2004) Organotellurium compound toxicity in a promyelocytic cell line compared to non-tellurium-containing organic analog. Toxicol In Vitro 18:475–482
Iwase K, Tatsuishi T, Nishimura Y, Yamaguchi JY, Oyama Y, Miyoshi N, Wada M (2004) Cytometric analysis of adverse action of diphenyl ditelluride on rat thymocytes: cell shrinkage as a cytotoxic parameter. Environ Toxicol 19:614–619
Nogueira CW, Rotta LN, Perry ML, Souza DO, da Rocha JB (2001) Diphenyl diselenide and diphenyl ditelluride affect the rat glutamatergic system in vitro and in vivo. Brain Res 906:157–163
Nogueira CW, Zeni G, Rocha JB (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 104:6255–6285
Stangherlin EC, Favero AM, Zeni G, Rocha JB, Nogueira CW (2005) Teratogenic vulnerability of Wistar rats to diphenyl ditelluride. Toxicology 207:231–239
Moretto MB, Funchal C, Zeni G, Rocha JB, Pessoa-Pureur R (2005) Organoselenium compounds prevent hyperphosphorylation of cytoskeletal proteins induced by the neurotoxic agent diphenyl ditelluride in cerebral cortex of young rats. Toxicology 210:213–222
Funchal C, Moretto MB, Vivian L, Zeni G, Rocha JB, Pessoa-Pureur R (2006) Diphenyl ditelluride- and methylmercury-induced hyperphosphorylation of the high molecular weight neurofilament subunit is prevented by organoselenium compounds in cerebral cortex of young rats. Toxicology 222:143–153
Borges VC, Rocha JB, Nogueira CW (2005) Effect of diphenyl diselenide, diphenyl ditelluride and ebselen on cerebral Na(+), K(+)-ATPase activity in rats. Toxicology 215:191–197
Nogueira CW, Borges VC, Zeni G, Rocha JB (2003) Organochalcogens effects on delta-aminolevulinate dehydratase activity from human erythrocytic cells in vitro. Toxicology 191:169–178
Laden BP, Porter TD (2001) Inhibition of human squalene monooxygenase by tellurium compounds: evidence of interaction with vicinal sulfhydryls. J Lipid Res 42:235–240
Lenardão EJ, Silva MS, Mendes SR, Azambuja F, Jacob RG, Santos PCS, Perin G (2007) Synthesis of β-phenylchalcogeno-α, β-unsaturated esters, ketones and nitriles using microwave and solvent-free conditions. J Braz Chem Soc 18:943–950
Comasseto JV, Ling LW, Petragnani N, Stefani HA (1997) Vinylic selenides and tellurides-preparation, reactivity a synthetic application. Synthesis 4:373
Goeger DE, Ganther HE (1994) Oxidation of dimethylselenide to dimethylselenoxide by microsomes from rat liver and lung and by flavin-containing monooxygenase from pig liver. Arch Biochem Biophys 310:448–451
Bjornstedt M, Odlander B, Kuprin S, Claesson HE, Holmgren A (1996) Selenite incubated with NADPH and mammalian thioredoxin reductase yields selenide, which inhibits lipoxygenase and changes the electron spin resonance spectrum of the active site iron. Biochemistry 35:8511–8516
Park HS, Park E, Kim MS, Ahn K, Kim IY, Choi EJ (2000) Selenite inhibits the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) through a thiol redox mechanism. J Biol Chem 275:2527–2531
Gupta N, Porter TD (2001) Inhibition of human squalene monooxigenase by selenium compounds. J Biochem Mol Toxicol 16:18–23
Chen F, Vallyathan V, Castranova V, Shi X (2001) Cell apoptosis induced by carcinogenic metals. Mol Cell Biochem 222:183–188
Halliwell BG, Gutteridge JMC (2007) Measurement of reactive species. Oxford University Press, Oxford, pp 268–340
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Diplock AT (1994) Antioxidants and free radical scavengers. Elsevier, Amsterdam, pp 113–130
Heffner JA, Repine RJ (1989) State of the art: pulmonary strategies of antioxidant defense. Am Rev Respir Dis 140:531–554
Alho H, Leinonen JS, Erhola M, Lonnrot K, Aejmelaeus R (1998) Assay of antioxidant capacity of human plasma and CSF in aging and disease. Restor Neurol Neurosci 12:159–165
Funchal C, Latini A, Jacques-Silva MC, Dos Santos AQ, Buzin L, Gottfried C, Wajner M, Pessoa-Pureur R (2006) Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Int 49:640–650
Petragnani N (1994) Tellurium in organic synthesis. Academic Press, New York
Zeni G, Braga AL, Stefani HA (2003) Palladium-catalyzed coupling of sp(2)-hybridized tellurides. Acc Chem Res 36:731–738
Zeni G, Ludtke DS, Panatieri RB, Braga AL (2006) Vinylic tellurides: from preparation to their applicability in organic synthesis. Chem Rev 106:1032–1076
Silveira CC, Braga AL, Guerra RB (2002) Stereoselective synthesis of alpha-phenylchalcogeno-alpha, beta-unsaturated esters. Tetrahedron Lett 43:3395–3397
Yamaguchi T, Takamura H, Matoba T, Terao J (1998) HPLC method for evaluation of the free radical-scavenging activity of foods by using 1, 1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem 62:1201–1204
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Reznick AZ, Packer L (1994) Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol 233:357–363
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:14114–14115
Marklund S (1985) Handbook of methods for oxygen radical research. CRC Press, Boca Raton
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Guthenberg C, Mannervik B (1981) Glutathione S-transferase (transferase pi) from human placenta is identical or closely related to glutathione S-transferase (transferase rho) from erythrocytes. Biochim Biophys Acta 661:255–260
Hevel JM, Marletta MA (1994) Nitric-oxide synthase assays. Methods Enzymol 233:250–258
Halliwell B, Gutteridge JM (1981) Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 128:347–352
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Maciel EN, Bolzan RC, Braga AL, Rocha JB (2000) Diphenyl diselenide and diphenyl ditelluride differentially affect delta-aminolevulinate dehydratase from liver, kidney, and brain of mice. J Biochem Mol Toxicol 14:310–319
Widy-Tyszkiewicz E, Piechal A, Gajkowska B, Smialek M (2002) Tellurium-induced cognitive deficits in rats are related to neuropathological changes in the central nervous system. Toxicol Lett 131:203–214
Vinson JA (1998) Flavonoids in foods as in vitro and in vivo antioxidants. Adv Exp Med Biol 439:151–164
Borges VC, Rocha JB, Savegnago L, Nogueira CW (2007) Repeated administration of diphenyl ditelluride induces hematological disorders in rats. Food Chem Toxicol 45:1453–1458
Avila DS, Gubert P, Dalla Corte CL, Alves D, Nogueira CW, Rocha JB, Soares FA (2007) A biochemical and toxicological study with diethyl 2-phenyl-2-tellurophenyl vinylphosphonate in a sub-chronic intraperitoneal treatment in mice. Life Sci 80:1865–1872
Tremaroli V, Fedi S, Zannoni D (2007) Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch Microbiol 187:127–135
Zugno AI, Stefanello FM, Scherer EB, Mattos C, Pederzolli CD, Andrade VM, Wannmacher CM, Wajner M, Dutra-Filho CS, Wyse AT (2008) Guanidinoacetate decreases antioxidant defenses and total protein sulfhydryl content in striatum of rats. Neurochem Res 33:1804–1810
Amici A, Levine RL, Tsai L, Stadtmans ER (1989) Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem 264:3341–3346
Yan LJ (2009) Analysis of oxidative modification of proteins. Curr Prot Cell Biol, Chap. 14, Unit 14.4
Doucet A (1988) Function and control of Na-K-ATPase in single nephron segments of the mammalian kidney. Kidney Int 34:749–760
Jorgensen PL (1986) Structure, function and regulation of Na, K-ATPase in the kidney. Kidney Int 29:10–20
Jaffe EK (1995) Porphobilinogen synthase, the first source of heme’s asymmetry. J Bioenerg Biomembr 27:169–179
Bechara EJH, Medeiros MHG, Monteiro HP, Hermes-Lima M, Pereira B, Demasi M, Costa C, Adballa DSP, Onuki J, Wendel CMA, Masci PD (1993) A free radical hypothesis of lead poisoning and in born porphyrias associated with 5-aminolevulinic overload. Quimica Nova 16:385–392
Emanuelli T, Pagel FW, Alves LB, Regner A, Souza DO (2001) Inhibition of adenylate cyclase activity by 5-aminolevulinic acid in rat and human brain. Neurochem Int 38:213–218
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Mates JM, Perez-Gomez C, Nunez de Castro I (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
Remacle J, Michiels C, Raes M (1992) The importance of antioxidant enzymes in cellular aging and degeneration. EXS 62:99–108
Kaur P, Yousuf S, Ansari MA, Siddiqui A, Ahmad AS, Islam F (2003) Tellurium-induced dose-dependent impairment of antioxidant status: differential effects in cerebrum, cerebellum, and brainstem of mice. Biol Trace Elem Res 94:247–258
Borsetti F, Tremaroli V, Michelacci F, Borghese R, Winterstein C, Daldal F, Zannoni D (2005) Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res Microbiol 156:807–813
Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS (1992) Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 298:431–437
Yamakura F, Matsumoto T, Ikeda K, Taka H, Fujimura T, Murayama K, Watanabe E, Tamaki M, Imai T, Takamori K (2005) Nitrated and oxidized products of a single tryptophan residue in human Cu, Zn-superoxide dismutase treated with either peroxynitrite-carbon dioxide or myeloperoxidase-hydrogen peroxide-nitrite. J Biochem 138:57–69
Halliwell B (1994) Free radicals and antioxidants: a personal view. Nutr Rev 52:253–265
Sies H (1991) Oxidative stress: from basic research to clinical application. Am J Med 91:31S–38S
Strayo D, Adhikari S, Tilak-Jain J, Menon VP, Devasagayam TPA (2008) Antioxidant activity of an aminothiazole compound: possible mechanisms. Chem-Biol Interact 173:215–223
Acknowledgment
This work was supported by Centro Universitário Metodista IPA and Universidade de Caxias do Sul.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvalho, C.A.S., Gemelli, T., Guerra, R.B. et al. Effect of in vitro exposure of human serum to 3-butyl-1-phenyl-2-(phenyltelluro)oct-en-1-one on oxidative stress. Mol Cell Biochem 332, 127–134 (2009). https://doi.org/10.1007/s11010-009-0182-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0182-6