Abstract
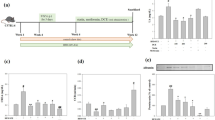
Diabetes and smoking have been considered as major health problems individually and their seriousness related to health hazard has been well reported. The role of nicotine in causing or worsening effect on diabetes is not well understood. The aim of our study was to investigate the effect of nicotine on experimental diabetes and to analyze the effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a bisdemethoxy curcumin analog (BDMCA) in streptozotocin and nicotine induced toxicity. Group I: control rats; Group II: nicotine (2.5 mg/kg b.wt); Group III: streptozotocin (STZ) (40 mg/kg b.wt); Group IV: STZ (40 mg/kg b.wt) + nicotine (2.5 mg/kg b.wt); Group V: STZ + nicotine + BDMCA (40 mg/kg b.wt); Group VI: STZ + nicotine + BDMCA (80 mg/kg b.wt). Efficacy of BDMCA was determined by evaluating blood glucose, thiobarbituric acid reactive substances (TBARS), hydroperoxides (HP), activities of marker enzymes alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) and activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). From our study, we have observed that nicotine not only aggravates diabetic complications but also increased the risk for diabetes. BDMCA, at a dose 80 mg/kg body weight was found to be more effective in decreasing toxic effects induced by nicotine and STZ.








Similar content being viewed by others
References
Baynes JW (1991) Role of oxidative stress in development of complication of diabetes. Diabetes 40(4):405–412. doi:10.2337/diabetes.40.4.405
Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free radic boil Med 10:339–352
Siemianowioz K, Gminski J, Telega A, Wojcik A, Posielenzna B, Grabowska-Bochenek R, Francuz T (2004) Blood antioxidant parameters in patients with diabetic retinopathy. Int J Mol Med 14:433–437
Yildiz D, Ercal N, Armstrong DW (1998) Nicotine enantiomers and oxidative stress. Toxicology 130:155–165. doi:10.1016/S0300-483X(98)00105-X
Newman MB, Arendash GW, Shytle RD, Bickford PC, Tighe PR, Sanberg PR (2002) Nicotine’s oxidative and antioxidant properties in CNS. Life Sci 71:2807–2820. doi:10.1016/S0024-3205(02)02135-5
Gillham B, Papachristodoulou DK, Thomas JH (1977) Wills’ biochemical basis of medicine, 3rd edn. Butterworth-Heinemann, Oxford, pp 343–354
Reddy S, Aggarwal BB (1994) Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett 341:19–22. doi:10.1016/0014-5793(94)80232-7
Cuvrelier ME, Richard H, Berset C (1992) Comparison of the antioxidant activity of some acid phenols, structure-activity relationship. Biosci Biotechnol Biochem 56:324–325
Jain SK, Rains J, Jones K (2006) Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic Biol Med 41:92–96. doi:10.1016/j.freeradbiomed.2006.03.008
Dinesh Babu KV, Rajasekaran KN (1994) Simplified conditions for the synthesis of curcumin I and other curcuminoids. Org Prep Proced Int 26:497–509
Kalpana C, Menon VP (2004) Modulatory effects of curcumin on lipid peroxidation and antioxidant status during nicotine-induced toxicity. Pol J Pharmacol 56:581–586
Siddique O, Sun Y, Lin K, Chien YW (1987) Facilitated transdermal transport of insulin. J Pharm Sci 76:341–345. doi:10.1002/jps.2600760416
Saski T, Matsy S, Sonae A (1972) Effect of acetic acid concentration on the colour reaction in the O-toluidine boric acid method for blood glucose estimation. Rinsho Kagaku 1:346–353
King EJ, Armstrong AR (1988) Calcium, phosphorus and phosphatase. In:Practical clinical biochemistry, CBS Publishers, New Delhi; p.458
Young DS, Pestaner LC, Gibberman V (1975) Effects of drugs on clinical laboratory tests. Clin Chem 21:1D–432D
Niehaus WG, Samuelsson B (1968) Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130. doi:10.1111/j.1432-1033.1968.tb00428.x
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion Fe2+ oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal Biochem 202:384–389. doi:10.1016/0003-2697(92)90122-N
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase (SOD). Indian J Biochem Biophys 21:130–132
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. doi:10.1016/0003-2697(72)90132-7
Rotruck JT, Pope AL, Ganther HE, Swason AB, Hafeman DG, Hoekstra WG (1973) Biochemical roles as a component of glutathione peroxidase. Science 179:588–590. doi:10.1126/science.179.4073.588
Yoshikawa H, Hellstrom-Lindahl E, Grill V (2005) Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism 54:247–254. doi:10.1016/j.metabol.2004.08.020
Crowley-Weber CL, Dvorakova K, Crowley C, Bernstein H, Bernstein C, Garewal H, Payne CM (2003) Nicotine increases oxidative stress, activates NF-kappaB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: relevance to colon carcinogenesis. Chem Biol Interact 145:53–56. doi:10.1016/S0009-2797(02)00162-X
Mahesh T, Balasubashini M, Menon V (2005) Effect of photo-irradiated curcumin treatment against oxidative stress in streptozotocin-induced diabetic rats. J Med Food 8:251–255. doi:10.1089/jmf.2005.8.251
Anusuya S, Menon VP, Viswanathan P, Rajasekaran KN (2003) Protection of pancreatic β-cell by the potential antioxidant bis-o-hydroxycinnamoyl methane analogue of natural curcuminoid in experimental diabetes. J Pharm Pharm Sci 6:327–333
Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ, Huang ZS, Chan AS (2006) Alpha-glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur J Med Chem 41:213–218. doi:10.1016/j.ejmech.2005.10.012
Anto RT, George J, Dinesh Babu KV, Rajasekaran KN, Kuttan R (1996) Antimutagenic and anticarcinogenic activity of natural and synthetic curcuminoids. Mutat Res 370:127–131. doi:10.1016/0165-1218(96)00074-2
Drent M, Cobben NAM, Henderson RF, Wouters EFM, Van Dieijen-Visser M (1996) Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir 9:1736–1742. doi:10.1183/09031936.96.09081736
Wetscher GJ, Bagchi D, Perdikis G, Bagchi M, Redmond EJ, Hinder PR, Glaser K, Hinder RA (1995) In vitro free radical production in rat oesophageal mucosa induced by nicotine. Dig Dis Sci 40:853–858. doi:10.1007/BF02064991
Sudheer AR, Kalpana C, Srinivasan M, Menon VP (2005) Ferrulic acid modulates altered lipid profiles and prooxidant antioxidant status in circulation during nicotine-induced toxicity: a dose dependent study. Toxicol Mech Methods 15:375–381. doi:10.1080/15376520500194783
Tunali S, Yanardag R (2006) Effect of Vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol Res 53(3):271–277. doi:10.1016/j.phrs.2005.12.004
Reddy AC, Lokesh BR (1992) Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem 111:117–124
Guan ZZ, Yu WF, Nordberg A (2003) Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int 43:243–249. doi:10.1016/S0197-0186(03)00009-3
Anwar MM, Meki AR (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A Mol Integr Physiol 135(4):539–547. doi:10.1016/S1095-6433(03)00114-4
Ramachandran B, Ravi K, Narayanan V, Kandaswamy M, Subramaniann S (2004) Effect of macrocyclic binuclear oxovanadium complex on tissue defense system in streptozotocin-induced diabetic rats. Clin Chim Acta 345:141–150. doi:10.1016/j.cccn.2004.03.014
Kalpana C, Menon VP, Rajasekharan KN (2005) Modulatory effects of curcumin and curcumin analog on circulatory lipid profile during nicotine-induced toxicity in wistar rats. J Med Food 8:246–250. doi:10.1089/jmf.2005.8.246
Ugochukwu NH, Mukes JD, Figgers CL (2006) Ameliorative effects of dietary caloric restriction on oxidative stress and inflammation in the brain of streptozotocin-induced diabetic rats. Clin Chim Acta 370:165–173. doi:10.1016/j.cca.2006.02.003
Avti PK, Kumar S, Pathak CM, Vaiphei K, Khanduja KL (2006) Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci 89:547–553. doi:10.1093/toxsci/kfj041
Cohen G, Hochstein P (1963) Glutathione peroxidase: the primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry 2:1420–1428. doi:10.1021/bi00906a038
Khopde SM, Priyadarsini KI, Venkatesan P, Rao MN (1999) Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys Chem 80:85–91
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, B.V., Sundari, J.S., Balamurugan, E. et al. Prevention of nicotine and streptozotocin treatment induced circulatory oxidative stress by bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in diabetic rats. Mol Cell Biochem 331, 127–133 (2009). https://doi.org/10.1007/s11010-009-0150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0150-1