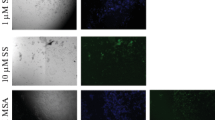
Abstract
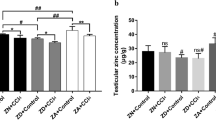
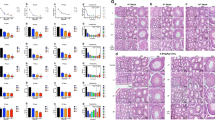
Selenium has been linked to cell survival and apoptosis. Apoptosis plays an important role in spermatogenesis. Evidence suggests that reactive oxygen species induce apoptotic pathways. Although the mechanism by which oxidants mediate apoptosis is not well defined, the mitogen-activated protein kinase (MAPK) and caspase pathways have been implicated in apoptosis. Thus, this study was designed, keeping in view the critical balance between cell proliferation and apoptosis for normal spermatogenesis, and the requirement of selenium for the maintenance of male fertility. The intracellular selenium status was modulated by feeding selenium-deficient and -excess diet for 8 weeks. Involvement of p38 MAPK and ROS was monitored. Apoptotic factors like caspases and Bcl-2 were also analyzed. It was observed that the selenium levels were altered along with an increase in ROS generation and lipid peroxidation. mRNA expression of p38, caspases 3, and 8 increased, whereas that for Bcl-2 decreased. Western immunoblot analysis and immunohistochemical localization studies for p38 showed a similar increase. Integrity of DNA was altered in the form of apoptotic cells. Thus, the results presented in this study suggest that sodium selenite causes apoptosis and the toxicity of selenite is mediated by increase in ROS. Morevoer, ROS generation is associated with increased expression of p38, caspases 3 and 8, and decreased Bcl-2 expression. Our data indicate that p38 participates in testicular apoptosis and that selenium is required for maintenance of the critical balance between cell death and proliferation.









Similar content being viewed by others
References
Borek C, Ong A, Mason H, Donahue L, Biaglow JE (1986) Selenium and vitamin E inhibit radiogenic and chemically induced transformation in vitro via different mechanisms. Proc Natl Acad Sci USA 83:1490–1494. doi:10.1073/pnas.83.5.1490
Zhuang S, Demirs JT, Kochevar IE (2000) p38 Mitogen-activated protein kinase mediates Bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem 275(34):25939–25948. doi:10.1074/jbc.M001185200
Behne D, Hilmert H, Scheid S, Gessner H, Elger W (1988) Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta 966(1):12–21
Shen HM, Yang DF, Ong DN (1999) Induction of oxidative stress and apoptosis in sodium selenite-treated human hepatoma cells (HepG2). Int J Cancer 81:820–828. doi:10.1002/(SICI)1097-0215(19990531)81:5<820::AID-IJC25>3.0.CO;2-F
Sundaram N, Pahwa AK, Ard MD, Lin N, Perkins E, Bowles AP Jr (2000) Selenium causes growth inhibition and apoptosis in human brain tumor cell lines. J Neurooncol 46:125–133. doi:10.1023/A:1006436326003
Redman C, Xu MJ, Peng YM, Scott JA, Payne C, Clark LC, Nelson MA (1997) Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells. Carcinogenesis 18:1195–1202. doi:10.1093/carcin/18.6.1195
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241. doi:10.1016/S0140-6736(00)02490-9
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333. doi:10.1016/S0891-5849(00)00302-6
Dumont A, Hehner SP, Hofmann TG, Ueffing M, Droge W, Schmitz ML (1999) Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene 18:747–757. doi:10.1038/sj.onc.1202325
Wang X, Sharma RK, Sikka SC, Thomas AJ Jr, Falcone T, Agarwal A (2003) Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril 80:531–535. doi:10.1016/S0015-0282(03)00756-8
Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME (1998) Apoptosis signaling by death receptors. Eur J Biochem 254:439–459. doi:10.1046/j.1432-1327.1998.2540439.x
Marshall CJ (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 4:82–89. doi:10.1016/0959-437X(94)90095-7
Kyriakis JM, Avruch J (1990) pp54 microtubule-associated protein 2 kinase, A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-l-lysine. J Biol Chem 265:17355–17363
Lee JC, Laydon JT, McDonnell PC, Gallagher TC, Kumar S, Green D et al (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–746. doi:10.1038/372739a0
Shalini S, Bansal MP (2005) Role of selenium in regulation of spermatogenesis: involvement of activator protein 1. Biofactors 23:151–162. doi:10.1002/biof.5520230304
Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A (1999) Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J Immunol 162:1692–1700
Raff M (1998) Cell suicide for beginners. Nature 396:119–122. doi:10.1038/24055
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316. doi:10.1126/science.281.5381.1312
Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J et al (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817–827. doi:10.1016/S0092-8674(00)81266-0
Choi WS, Eom DS, Han BS, Kim WK, Han BH, Choi EJ et al (2004) Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. J Biol Chem 279:20451–20460. doi:10.1074/jbc.M311164200
Boesen-de Cock JG, Tepper AD, de Vries E, van Blitterswijk WJ, Borst J (1999) Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and γ-radiation downstream from caspase-8 activation. J Biol Chem 274:14255–14261. doi:10.1074/jbc.274.20.14255
Reed JC (1994) Bcl-2 and the regulation of programmed cell death. J Cell Biol 124:1–6. doi:10.1083/jcb.124.1.1
Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P (1997) An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 16:2262–2270. doi:10.1093/emboj/16.9.2262
Burk RF (1987) Production of selenium deficiency in rat. Methods Enzymol 143:307–313. doi:10.1016/0076-6879(87)43058-9
Hasunuma R, Ogawi T, Kawaniska Y (1982) Fluorometric determination of selenium in nanogram amounts in biological materials using 2,3-diaminonaphthalene. Anal Biochem 126:242–245. doi:10.1016/0003-2697(82)90510-3
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–168
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99(3):667–676
Driver AS, Rao P, Kodavanti S, Mundy WR (2000) Age-related changes in the reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. doi:10.1016/S0892-0362(99)00069-0
Shalini S, Bansal MP (2007) Alterations in selenium status influences reproductive potential of male mice by modulation of transcription factor NFκB. Biometals 20:49–59. doi:10.1007/s10534-006-9014-2
Hafeman DG, Sunde RA, Hoekstra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104:580–587
Surai P, Kostjuk L, Wishart G, Macpherson A, Speake B, Noble R et al (1998) Effect of vitamin E and selenium supplementation of cockerel diets on glutathione peroxidase activity and lipid peroxidation susceptibility in sperm, testes and liver. Biol Trace Elem Res 64:119–132. doi:10.1007/BF02783329
Shen HM, Yang CF, Liu J, Ong CN (2001) Superoxide radical initiated apoptotic signaling pathway in selenite treated HEPR2 cells: mitochondria serve as the main target. Free Radic Biol Med 30:9–21. doi:10.1016/S0891-5849(00)00421-4
Sen CK, Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10:709–720
Spallholz JE (2001) Selenium and the prevention of cancer. Part II: mechanism for the carcinostatic activity of Se compounds, Bull STDA: 1–12
Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M et al (2003) H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activation JNKs and p38 MAPK. Exp Neurol 180:144–155. doi:10.1016/S0014-4886(02)00059-6
Sinha Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH et al (2003) Key apoptotic pathways for heat-induced programmed cell death in the testis. Endocrinology 144(7):3167–3175. doi:10.1210/en.2003-0175
Nolan Y, Verker E, Lynch AM, Lynch MA (2003) Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Exp Neurol 184:794–804. doi:10.1016/S0014-4886(03)00301-7
Paasch U, Grunewald S, Fitzl G, Glander H (2003) Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J Androl 24:246–252
Song JJ, Lee YJ (2004) Daxx deletion mutant (amino acids 501–625)-induced apoptosis occurs through the JNK/p38-Bax-dependent mitochondrial pathway. J Cell Biochem 92:1257–1270. doi:10.1002/jcb.20155
Bhattacharyya A, Pathak S, Basak C, Law S, Kundu M, Basu J (2003) Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8. J Biol Chem 278:26517–26525. doi:10.1074/jbc.M300852200
Denial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219. doi:10.1016/S0092-8674(04)00046-7
Lee VY, McClintock DS, Santore MT, Budinger GR, Chandel NS (2002) Hypoxia sensitizes cells to nitric oxide-induced apoptosis. J Biol Chem 277:16067–16074. doi:10.1074/jbc.M111177200
Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM (1993) Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361:739–742. doi:10.1038/361739a0
Grimm C, Wenzel A, Hafezi F, Reme’ CE (2000) Gene expression in the mouse retina: the effect of damaging light. Mol Vis 6:252–260
Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K (1996) Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia 39:1306–1312. doi:10.1007/s001250050574
Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B et al (2003) Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci PNAS 100:7797–7802. doi:10.1073/pnas.1330920100
Acknowledgment
The authors acknowledge the financial support provided by Life Science Research Board, DRDO, Govt of India, New Delhi (India).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranawat, P., Bansal, M.P. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: implications in spermatogenesis. Mol Cell Biochem 330, 83–95 (2009). https://doi.org/10.1007/s11010-009-0103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0103-8