Abstract
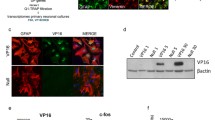
Astrocytes play a more important role than simply providing physical support for neurons, however, the function(s) of type 1 and type 2 astrocytes (T1As, T2As), remains unclear. A DNA microarray was used to identify gene expression in cultured T1As and T2As isolated from postnatal day 1 rat cortex. Ninety-nine of the 138 differentially expressed genes were involved in a diverse number of processes. The fasciculation and elongation protein zeta-1 (FEZ1) gene was studied further because it has been suggested that it is not expressed by astrocytes. RT-PCR and Western blots confirmed the microarray data and showed that FEZ1 was present in T1 and T2As and is more highly expressed in T2As. Immunocytochemistry revealed that FEZ1 was located in the astrocytic cytoplasm and cell processes but not the nucleus. The results contribute to a clearer understanding of the two types of astrocytes.



Similar content being viewed by others
References
Wang LC, Baird DH, Hatten ME et al (1994) Astroglial differentiation is required for support of neurite outgrowth. J Neurosci 14:3195–3207
Alexander JK, Fuss B, Colello RJ (2006) Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol 2:93–103. doi:10.1017/S1740925X0600010X
Raff MC, Ffrench-Constant C, Miller RH (1987) Glial cells in the rat optic nerve and some thoughts on remyelination in the mammalian CNS. J Exp Biol 132:35–41
Raff MC, Abney ER, Cohen J et al (1983) Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci 3:1289–1300
Yan M, Xia C, Niu S et al (2008) The role of TNF-alpha and its receptors in the production of beta-1, 4-galactosyltransferase I mRNA by rat primary type-2 astrocytes. Cell Mol Neurobiol 28:223–236. doi:10.1007/s10571-007-9182-9
Miller RH, Raff MC (1984) Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4:585–592
Raff MC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390–396. doi:10.1038/303390a0
Murakami K, Asou H, Adachi T et al (1999) Neutral glycolipid and ganglioside composition of type-1 and type-2 astrocytes from rat cerebral hemisphere. J Neurosci Res 55:382–393. doi:10.1002/(SICI)1097-4547(19990201)55:3<382::AID-JNR13>3.0.CO;2-M
Raff MC, Abney ER, Miller RH (1984) Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol 106:53–60. doi:10.1016/0012-1606(84)90060-5
Bloom L, Horvitz HR (1997) The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci USA 94:3414–3419. doi:10.1073/pnas.94.7.3414
Kuroda S, Nakagawa N, Tokunaga C et al (1999) Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol 144:403–411. doi:10.1083/jcb.144.3.403
Suzuki T, Okada Y, Semba S et al (2005) Identification of FEZ1 as a protein that interacts with JC virus agnoprotein and microtubules: role of agnoprotein-induced dissociation of FEZ1 from microtubules in viral propagation. J Biol Chem 280:24948–24956. doi:10.1074/jbc.M411499200
Ikuta J, Maturana A, Fujita T et al (2007) Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem Biophys Res Commun 353:127–132. doi:10.1016/j.bbrc.2006.11.142
Fujita T, Maturana AD, Ikuta J et al (2007) Axonal guidance protein FEZ1 associates with tubulin and kinesin motor protein to transport mitochondria in neurites of NGF-stimulated PC12 cells. Biochem Biophys Res Commun 361:605–610. doi:10.1016/j.bbrc.2007.07.050
Assmann EM, Alborghetti MR, Camargo ME et al (2006) FEZ1 dimerization and interaction with transcription regulatory proteins involves its coiled-coil region. J Biol Chem 281:9869–9881. doi:10.1074/jbc.M513280200
Honda A, Miyoshi K, Baba K et al (2004) Expression of fasciculation and elongation protein zeta-1 (FEZ1) in the developing rat brain. Brain Res Mol Brain Res 122:89–92. doi:10.1016/j.molbrainres.2003.11.020
Carlson DJ, Strait KA, Schwartz HL et al (1996) Thyroid hormone receptor isoform content in cultured type 1 and type 2 astrocytes. Endocrinology 137:911–917. doi:10.1210/en.137.3.911
McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902. doi:10.1083/jcb.85.3.890
Jianghong H, Ye Z, Chunlin X et al (2008) Expression of fasciculation and elongation protein zeta-1 in type 1 and type 2 astrocytes. Acta Anat Sin 39:636–639
Miller RH, Abney ER, David S et al (1986) Is reactive gliosis a property of a distinct subpopulation of astrocytes? J Neurosci 6:22–29
Janzer RC, Raff MC (1987) Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325:253–257. doi:10.1038/325253a0
Tan L, Sun S, Duan S et al (2005) Regulation of astroglia on synaptic plasticity in the CA1 region of rat hippocampus. J Huazhong Univ Sci Technolog Med Sci 25:484–487
Jimenez AI, Castro E, Mirabet M et al (1999) Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia 26:119–128. doi:10.1002/(SICI)1098-1136(199904)26:2<119::AID-GLIA3>3.0.CO;2-D
Chen G, Li HM, Chen YR et al (2007) Decreased estradiol release from astrocytes contributes to the neurodegeneration in a mouse model of Niemann–Pick disease type C. Glia 55:1509–1518. doi:10.1002/glia.20563
Ffrench-Constant C, Raff MC (1986) The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. Nature 323:335–338. doi:10.1038/323335a0
Hollander H, Makarov F, Dreher Z et al (1991) Structure of the macroglia of the retina: sharing and division of labour between astrocytes and Muller cells. J Comp Neurol 313:587–603. doi:10.1002/cne.903130405
Sims TJ, Waxman SG, Black JA et al (1985) Perinodal astrocytic processes at nodes of Ranvier in developing normal and glial cell deficient rat spinal cord. Brain Res 337:321–331. doi:10.1016/0006-8993(85)90069-1
Blasius TL, Cai D, Jih GT et al (2007) Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol 176:11–17. doi:10.1083/jcb.200605099
Koushika SP (2008) “JIP”ing along the axon: the complex roles of JIPs in axonal transport. Bioessays 30:10–14. doi:10.1002/bies.20695
Hackney DD, Stock MF (2008) Kinesin tail domains and Mg2+ directly inhibit release of ADP from head domains in the absence of microtubules. Biochemistry 47:7770–7778. doi:10.1021/bi8006687
Skene JH, Virag I (1989) Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol 108:613–624. doi:10.1083/jcb.108.2.613
Aigner L, Caroni P (1995) Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J Cell Biol 128:647–660. doi:10.1083/jcb.128.4.647
Denny JB (2006) Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr Neuropharmacol 4:293–304. doi:10.2174/157015906778520782
Kalil K, Skene JH (1986) Elevated synthesis of an axonally transported protein correlates with axon outgrowth in normal and injured pyramidal tracts. J Neurosci 6:2563–2570
Meiri KF, Pfenninger KH, Willard MB (1986) Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA 83:3537–3541. doi:10.1073/pnas.83.10.3537
Hoffman PN (1989) Expression of GAP-43, a rapidly transported growth-associated protein, and class II beta tubulin, a slowly transported cytoskeletal protein, are coordinated in regenerating neurons. J Neurosci 9:893–897
Vitkovic L, Steisslinger HW, Aloyo VJ et al (1988) The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes. Proc Natl Acad Sci USA 85:8296–8300. doi:10.1073/pnas.85.21.8296
Sensenbrenner M, Lucas M, Deloulme JC (1997) Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J Mol Med 75:653–663. doi:10.1007/s001090050149
Deloulme JC, Janet T, Au D et al (1990) Neuromodulin (GAP43): a neuronal protein kinase C substrate is also present in 0–2A glial cell lineage. Characterization of neuromodulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigen. J Cell Biol 111:1559–1569. doi:10.1083/jcb.111.4.1559
da Cunha A, Vitkovic L (1990) Regulation of immunoreactive GAP-43 expression in rat cortical macroglia is cell type specific. J Cell Biol 111:209–215. doi:10.1083/jcb.111.1.209
McKay RD (1989) The origins of cellular diversity in the mammalian central nervous system. Cell 58:815–821. doi:10.1016/0092-8674(89)90934-3
Jeffrey PL, Balcar VJ, Tolhurst O et al (2003) Avian Purkinje neuronal cultures: extrinsic control of morphology by cell type and glutamate. Methods Cell Biol 71:89–109. doi:10.1016/S0091-679X(03)01006-9
Lillien LE, Sendtner M, Raff MC (1990) Extracellular matrix-associated molecules collaborate with ciliary neurotrophic factor to induce type-2 astrocyte development. J Cell Biol 111:635–644. doi:10.1083/jcb.111.2.635
Hochstim C, Deneen B, Lukaszewicz A et al (2008) Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133:510–522. doi:10.1016/j.cell.2008.02.046
Liao H, Huang W, Schachner M et al (2008) Beta 1 integrin-mediated effects of tenascin-R domains EGFL and FN6–8 on neural stem/progenitor cell proliferation and differentiation in vitro. J Biol Chem 283:27927–27936. doi:10.1074/jbc.M804764200
Acknowledgments
This study was supported by National Natural Science Foundation of China (Grant No. 30772242). The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, J., Liu, J., Zhang, Z. et al. Expression of fasciculation and elongation protein zeta-1 (FEZ1) in cultured rat neonatal astrocytes. Mol Cell Biochem 325, 159–167 (2009). https://doi.org/10.1007/s11010-009-0030-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0030-8