Abstract
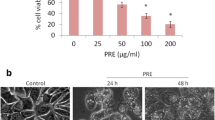
Mitochondria and associated oxidative stress have been shown to play critical roles in apoptotic death induced by various stress agents. Previously, we reported the antitumor property of diospyrin (D1), a plant-derived bisnaphthoquinonoid, and its diethylether derivative (D7), which was found to cause apoptotic death in human cancer cell lines. The present study aims to explore the relevant mechanism of apoptosis involving generation of cellular reactive oxygen species (ROS) by D7 in human breast carcinoma (MCF-7) cells. It was found that while D7 inhibited the proliferation of tumor cells, the associated apoptosis induced by D7 was prevented by treating the cells with N-acetyl-l-cysteine (NAC), an antioxidant, and cyclosporine A (CsA), an inhibitor of mitochondrial permeability transition (MPT). Experiments using suitable inhibitors also demonstrated that D7 could alter the electron flow in mitochondrial electron transport chain by affecting target(s) between complex I and complex III, and indicated the probable site of D7-induced generation of ROS. These results were further supported by confocal microscopic observation on changes in mitochondrial organization and shape in cells treated with D7. Taken together, the results of our study clearly suggested that the apoptosis induced by D7 would involve alteration of MPT, cardiolipin peroxidation, migration of Bax from cytosol to mitochondria, decreased expression of Bcl-2, and release of cytochrome c, indicating oxidative mechanism at the mitochondrial level in the tumor cells.






Similar content being viewed by others
References
O’Brien PJ (1991) Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact 80:1–41. doi:10.1016/0009-2797(91)90029-7
Powis G (1989) Free radical formation by antitumor quinones. Free Radic Biol Med 6:63–101. doi:10.1016/0891-5849(89)90162-7
Mohan IK, Kumar KV, Naidu MU et al (2006) Protective effect of CardiPro against doxorubicin-induced cardiotoxicity in mice. Phytomedicine 13:222–229. doi:10.1016/j.phymed.2004.09.003
Kovacic P (2003) Mechanism of drug and toxic actions of gossypol: focus on reactive oxygen species and electron transfer. Curr Med Chem 10:2711–2718. doi:10.2174/0929867033456369
Li CJ, Wang C, Pardee AB (1995) Induction of apoptosis by beta-lapachone in human prostate cancer cells. Cancer Res 55:3712–3715
Srinivas P, Gopinath G, Banerji A et al (2004) Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog 40:201–211. doi:10.1002/mc.20031
Chakrabarty S, Roy M, Hazra B et al (2002) Induction of apoptosis in human cancer cell lines by diospyrin, a plant-derived bisnaphthoquinonoid, and its synthetic derivatives. Cancer Lett 188:85–93. doi:10.1016/S0304-3835(02)00494-9
Hazra B, Kumar B, Biswas S et al (2005) Enhancement of the tumor inhibitory activity, in vivo, of diospyrin, a plant-derived quinonoid, through liposomal encapsulation. Toxicol Lett 157:109–117. doi:10.1016/j.toxlet.2005.01.016
Hazra B, Sur P, Roy DK et al (1984) Biological activity of diospyrin towards Ehrlich ascites carcinoma in Swiss A mice. Planta Med 50:295–297. doi:10.1055/s-2007-969713
Das Sarma M, Ghosh R, Patra A et al (2007) Synthesis and antiproliferative activity of some novel derivatives of diospyrin, a plant-derived naphthoquinonoid. Bioorg Med Chem 15:3672–3677. doi:10.1016/j.bmc.2007.03.050
Das Sarma M, Patra A, Chowdhury R, Chaudhuri K, Hazra B (2007) Novel glycoconjugates of diospyrin, a quinonoid plant product: synthesis and evaluation of cytotoxicity against human malignant melanoma (A375) and laryngeal carcinoma (Hep 2). Org Biomol Chem 5:3115–3125. doi:10.1039/b707851j
Hazra B, Das Sarma M, Kumar B et al (2007) Cytotoxicity of diospyrin and its derivatives in relation to the generation of reactive oxygen species in tumour cells in vitro and in vivo. Chemotherapy 53:173–176
Kumar B, Joshi J, Kumar A et al (2007) Radiosensitization by diospyrin diethylether in MCF-7 breast carcinoma cell line. Mol Cell Biochem 304:287–296. doi:10.1007/s11010-007-9511-9
Kumar B, Kumar A, Pandey BN, Hazra B, Mishra KP (2008) Enhancement of radiation-induced cytotoxicity in fibrosarcoma tumor in murine models, as well as in a human cell line, by diospyrin diethylether. Int J Radiat Biol 84:429–440. doi:10.1080/09553000802030736
Armstrong JS (2007) Mitochondrial medicine: pharmacological targeting of mitochondria in disease. Br J Pharmacol 151:1154–1165. doi:10.1038/sj.bjp.0707288
Galluzzi L, Larochette N, Zamzami N et al (2006) Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 25:4812–4830. doi:10.1038/sj.onc.1209598
Pilkington GJ, Parker K, Murray SA (2008) Approaches to mitochondrially mediated cancer therapy. Semin Cancer Biol 18:226–235. doi:10.1016/j.semcancer.2007.12.006
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Pandey BN, Sarma HD, Shukla D, Mishra KP (2006) Low dose radiation induced modification of reactive oxygen species and apoptosis in thymocytes of whole body irradiated mice. Int J Low Radiat 2:111–118. doi:10.1504/IJLR.2006.007901
Cai J, Jones DP (1998) Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273:11401–11404. doi:10.1074/jbc.273.19.11401
Pandey BN, Gordon DM, De Toledo SM et al (2006) Normal human fibroblasts exposed to high- or low-dose ionizing radiation: differential effects on mitochondrial protein import and membrane potential. Antioxid Redox Signal 8:1253–1261. doi:10.1089/ars.2006.8.1253
Russell JW, Golovoy D, Vincent AM et al (2002) High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16:1738–1748. doi:10.1096/fj.01-1027com
Wharton DC, Tzagoloff A (1967) Cytochrome oxidase from beef heart mitochondria. Methods Enzymol 10:245–250. doi:10.1016/0076-6879(67)10048-7
Li N, Ragheb K, Lawler G et al (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525. doi:10.1074/jbc.M210432200
Yang JC, Cortopassi GA (1998) Induction of the mitochondrial permeability transition causes release of the apoptogenic factor cytochrome c. Free Radic Biol Med 24:624–631. doi:10.1016/S0891-5849(97)00367-5
Nomura K, Imai T, Kobayashi T, Nakagawa Y (2000) Mitochondrial phospholipids hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J 351:183–193. doi:10.1042/0264-6021:3510183
Richter C (1998) Oxidative stress, mitochondria, and apoptosis. Restor Neurol Neurosci 12:59–62
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Trudel S, Paquet MR, Grinstein S (1991) Mechanism of vanadate-induced activation of tyrosine phosphorylation and of the respiratory burst in HL60 cells. Role of reduced oxygen metabolites. Biochem J 276(Pt 3):611–619
Vanden Hoek TL, Becker LB, Shao Z et al (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273:18092–18098. doi:10.1074/jbc.273.29.18092
Wolvetang EJ, Johnson KL, Krauer K et al (1994) Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett 339:40–44. doi:10.1016/0014-5793(94)80380-3
Nohl H, Jordan W (1986) The mitochondrial site of superoxide formation. Biochem Biophys Res Commun 138:533–539. doi:10.1016/S0006-291X(86)80529-0
Taylor DE, Ghio AJ, Piantadosi CA (1995) Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys 316:70–76. doi:10.1006/abbi.1995.1011
Takuma K, Yao J, Huang J et al (2005) ABAD enhances A β-induced cell stress via mitochondrial dysfunction. FASEB J 19:597–598
Halestrap AP, McStay GP, Clarke SJ (2002) The permeability transition pore complex: another view. Biochimie 84:153–166. doi:10.1016/S0300-9084(02)01375-5
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12:835–840. doi:10.1007/s10495-006-0525-7
Hoch FL (1992) Cardiolipins and biomembrane function. Biochim Biophys Acta 1113:71–133
Acknowledgments
This study was supported by the Board of Radiation and Nuclear Sciences, Department of Atomic Energy, Mumbai (Research Grant No. 2002/37/19/BRNS), and the University Grants Commission, New Delhi. Authors would like to acknowledge the technical assistance of Mr. Manjoor Ali, Radiation Biology and Health Sciences Division, BARC, Mumbai in the acquisition of images in Confocal Microscopy. The authors are grateful to Dr. Nandedkar, National Institute for Research in Reproductive Health, Parel, Mumbai, for providing the facility for carrying out the experiments on FACS analysis, and Mr. Akhilesh Chaurasia, SRF, CSIR, BARC for his help in fluorescence microscopy.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, B., Kumar, A., Pandey, B.N. et al. Role of mitochondrial oxidative stress in the apoptosis induced by diospyrin diethylether in human breast carcinoma (MCF-7) cells. Mol Cell Biochem 320, 185–195 (2009). https://doi.org/10.1007/s11010-008-9920-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9920-4