Abstract
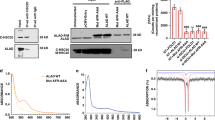
5-Aminolevulinic acid synthase 1 (ALAS1) is the first and rate-controlling enzyme of heme biosynthesis. This study was to determine the effects of heme and selected nonheme metalloporphyrins on human ALAS1 gene expression in hepatocytes. We found that, upon heme and cobalt protoporphyrin (CoPP) treatments, ALAS1 mRNA levels were down-regulated significantly by ca. 50% or more. Measurement of mRNA in the presence of actinomycin D showed that these down-regulations were due to the decreases in mRNA half-lives. Furthermore, the levels of mitochondrial mature ALAS1 protein were down-regulated by 60–70%, but those of the cytosolic precursor protein were up-regulated by 2–5-fold. Measurement of protein in the presence of cycloheximide (CHX) suggests that elevation of the precursor form is due to the increase in protein half-lives. These results provide novel insights into the mechanisms of heme repressional effects on ALAS1 and provide a rationale for further investigation of CoPP as a therapeutic agent for acute porphyric syndromes.







Similar content being viewed by others
Abbreviations
- ALAS1:
-
5-Aminolevulinic acid synthase 1
- ALAS2:
-
5-Aminolevulinic acid synthase 2
- CHX:
-
Cycloheximide
- CoPP:
-
Cobalt protoporphyrin
- CrMP:
-
Chromium mesoporphyrin
- GAPDH:
-
Glyceraldehyde phosphate dehydrogenase
- HO-1:
-
Heme oxygenase 1
- LMH:
-
Leghorn male hepatoma
- DMSO:
-
Dimethyl sulfoxide
- MnPP:
-
Manganese protoporphyrin
- PCR:
-
Polymerase chain reaction
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PBST:
-
Phosphate-buffered saline Tween-20
- PVDF:
-
Polyvinylidene fluoride
- SnMP:
-
Tin mesoporphyrin
- ZnMP:
-
Zinc mesoporphyrin
- ZnPP:
-
Zinc protoporphyrin
References
Ades IZ (1990) Heme production in animal tissues: the regulation of biogenesis of delta-aminolevulinate synthase. Int J Biochem 22:565–578. doi:10.1016/0020-711X(90)90032-X
Bonkovsky HL, Obando JV (1999) Role of HFE gene mutations in liver diseases other than hereditary hemochromatosis. Curr Gastroenterol Rep 1:30–37. doi:10.1007/s11894-999-0084-5
Reichheld JH, Katz E, Banner BF, Szymanski IO, Saltzman JR, Bonkovsky HL (1999) The value of intravenous heme-albumin and plasmapheresis in reducing postoperative complications of orthotopic liver transplantation for erythropoietic protoporphyria. Transplantation 67:922–928. doi:10.1097/00007890-199903270-00023
Bonkovsky HL, Healey JF, Lourie AN, Gerron GG (1991) Intravenous heme-albumin in acute intermittent porphyria: evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am J Gastroenterol 86(8):1050–1056
Riddle RD, Yamamoto M, Engel JD (1989) Expression of delta-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci USA 86:792–796. doi:10.1073/pnas.86.3.792
Bishop DF, Henderson AS, Astrin KH (1990) Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome. Genomics 7:207–214. doi:10.1016/0888-7543(90)90542-3
Sassa S, Nagai T (1996) The role of heme in gene expression. Int J Hematol 63:167–178. doi:10.1016/0925-5710(96)00449-5
Srivastava G, Borthwick IA, Maguire DJ, Elferink CJ, Bawden MJ, Mercer JF et al (1988) Regulation of 5-aminolevulinate synthase mRNA in different rat tissues. J Biol Chem 263:5202–5209
Yamamoto M, Kure S, Engel JD, Hiraga K (1988) Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J Biol Chem 263:15973–15979
Kolluri S, Sadlon TJ, May BK, Bonkovsky HL (2005) Haem repression of the housekeeping 5-Aminolevulinic acid synthase gene in the hepatoma cell line LMH. Biochem J 392:173–180. doi:10.1042/BJ20050354
Hamilton JW, Bement WJ, Sinclair PR, Sinclair JF, Alcedo JA, Wetterhahn KE (1991) Heme regulates hepatic 5-aminolevulinate synthase mRNA expression by decreasing mRNA half-life and not by altering its rate of transcription. Arch Biochem Biophys 289:387–392. doi:10.1016/0003-9861(91)90428-L
Cable EE, Cable JW, Bonkovsky HL (1993) Repression of hepatic delta-aminolevulinate synthase by heme and metalloporphyrins: relationship to inhibition of heme oxygenase. Hepatology 18:119–127
Cable EE, Pepe JA, Karamitsios NC, Lambrecht RW, Bonkovsky HL (1994) Differential effects of metalloporphyrins on messenger RNA levels of delta-aminolevulinate synthase and heme oxygenase Studies in cultured chick embryo liver cells. J Clin Invest 94:649–654. doi:10.1172/JCI117381
Drew PD, Ades IZ (1989) Regulation of the stability of chicken embryo liver delta-aminolevulinate synthase mRNA by hemin. Biochem Biophys Res Commun 162:102–107. doi:10.1016/0006-291X(89)91968-2
Cable EE, Miller TG, Isom HC (2000) Regulation of heme metabolism in rat hepatocytes and hepatocyte cell lines: delta-aminolevulinic acid synthase and heme oxygenase are regulated by different heme-dependent mechanisms. Arch Biochem Biophys 384:280–295. doi:10.1006/abbi.2000.2117
Dailey TA, Woodruff JH, Dailey HA (2005) Examination of mitochondrial protein targeting of haem synthetic enzymes: in vivo identification of three functional haem-responsive motifs in 5-aminolaevulinate synthase. Biochem J 386:381–386. doi:10.1042/BJ20040570
Munakata H, Sun JY, Yoshida K, Nakatani T, Honda E, Hayakawa S et al (2004) Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J Biochem 136:233–238. doi:10.1093/jb/mvh112
Yamamoto M, Hayashi N, Kikuchi G (1983) Translational inhibition by heme of the synthesis of hepatic delta-aminolevulinate synthase in a cell-free system. Biochem Biophys Res Commun 115:225–231. doi:10.1016/0006-291X(83)90993-2
Hayashi N, Watanabe N, Kikuchi G (1983) Inhibition by hemin of in vitro translocation of chicken liver delta-aminolevulinate synthase into mitochondria. Biochem Biophys Res Commun 115:700–706. doi:10.1016/S0006-291X(83)80201-0
Bonkovsky HL (1987) Porphyria: practical advice for the clinical gastroenterologist and hepatologist. Dig Dis 5:179–192
Bloomer JR, Bonkovsky HL (1989) The porphyrias. Dis Mon 35:1–54. doi:10.1016/0011-5029(89)90003-5
Simionatto CS, Cabal R, Jones L, Galbraith RA (1988) Thrombophlebitis and disturbed hemostasis following administration of intravenous hematin in normal volunteers. Am J Med 85:538–540
Goetsch CA, Bissell DM (1986) Instability of hematin used in the treatment of hepatic porphyria. N Engl J Med 315:235–238
Morris DL, Dudley MD, Pearson RD (1981) Coagulopathy associated with hematin treatment for acute intermittent porphyria. Ann Intern Med 95:700–701
Peterson JM, Pierach CA (1984) Hematin induced hemolysis in acute porphyria. Ann Intern Med 101:877
Dhar GJ, Bossenmaier I, Cardinal R, Petryka ZJ, Watson CJ (1978) Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Med Scand 203:437–443
Khanderia U (1986) Circulatory collapse associated with hemin therapy for acute intermittent porphyria. Clin Pharm 5:690–692
Bonkovsky HL (1993) Advances in understanding and treating ‘the little imitator’, acute porphyria. Gastroenterology 105:590–594
Cable EE, Gildemeister OS, Pepe JA, Donohue SE, Lambrecht RW, Bonkovsky HL (1996) Hepatic 5-aminolevulinic acid synthase mRNA stability is modulated by inhibitors of heme biosynthesis and by metalloporphyrins. Eur J Biochem 240:112–117. doi:10.1111/j.1432-1033.1996.0112h.x
Russo SM, Pepe JA, Cable EE, Lambrecht RW, Bonkovsky HL (1994) Repression of ALA synthase by heme and zinc-mesoporphyrin in a chick embryo liver cell culture model of acute porphyria. Eur J Clin Invest 24:406–415. doi:10.1111/j.1365-2362.1994.tb02184.x
Bonkovsky HL (1990) Porphyrin and heme metabolism and the porphyrias. In: Zakim D, Boyer TD (eds) Hepatology a textbook of liver disease, 2nd edn. Saunders Philadelphia, pp 378–424
Sinclair PR, Granick S (1975) Heme control on the synthesis of delta-aminolevulinic acid synthetase in cultured chick embryo liver cells. Ann NY Acad Sci 244:509–520. doi:10.1111/j.1749-6632.1975.tb41551.x
Granick S, Sinclair P, Sassa S, Grieninger G (1975) Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem 250:9215–9225
Shan Y, Lambrecht RW, Bonkovsky HL (2004) Identification of key elements that are responsible for heme-mediated induction of the avian heme oxygenase-1 gene. Biochim Biophy Acta 1679:87–94
Shan Y, Lambrecht RW, Ghaziani T, Donohue SE, Bonkovsky HL (2004) Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAs. J Biol Chem 279:51769–51774. doi:10.1074/jbc.M409463200
Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL (2006) Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J 20:2651–2653. doi:10.1096/fj.06-6346fje
Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R et al (2006) HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J Hepatol 45:5–12. doi:10.1016/j.jhep.2005.12.020
Zheng J, Yang X, Harrell JM, Ryzhikov S, Shim EH, Lykke-Andersen K et al (2002) CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Mol Cell 10:1519–1526. doi:10.1016/S1097-2765(02)00784-0
Yamauchi K, Hayashi N, Kikuchi G (1980) Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem 255:1746–1751
Igarashi J, Hayashi N, Kikuchi G (1978) Effects of administration of cobalt chloride and cobalt protoporphyrin on delta-aminolevulinate synthase in rat liver. J Biochem 84:997–1000
Gardner FH (1953) The use of cobaltous chloride in the anemia associated with chronic renal disease. J Lab Clin Med 41:182–198
Galbraith RA, Kappas A (1991) Cobalt protoporphyrin regulates body weight in beagle dogs: induction of weight loss in normal animals of stable adult weight. Pharmacology 43:96–105. doi:10.1159/000138834
Galbraith RA, Kappas A (1991) Intracerebroventricular administration of cobalt protoporphyrin elicits prolonged weight reduction in rats. Am J Physiol 261:R1395–R1401
Galbraith RA, Kappas A (1991) Regulation of food intake and body weight in rats by the synthetic heme analogue cobalt protoporphyrin. Am J Physiol 261:R1388–R1394
Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH et al (2005) Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 122:505–515. doi:10.1016/j.cell.2005.06.040
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735
Bonkowsky HL, Collins A, Doherty HM, Tschudy DP (1973) The glucose effect in rat liver: studies of delta-aminolaevulinate synthase and tyrosine aminotransferase. Biochim Biophys Acta 320:561–576
Green D, Reynolds N, Klein J, Kohl H, Ts’ao CH (1983) The inactivation of hemostatic factors by hematin. J Lab Clin Med 102:361–369
Jones RL (1986) Hematin-derived anticoagulant Generation in vitro and in vivo. J Exp Med 163:724–739. doi:10.1084/jem.163.3.724
Acknowledgments
This work was supported by United States Public Health Service Grants RO1-DK38825 and contracts NO-1 DK92326 and UO-1 DK065193. Authors thank Dr. Henry M. Furneaux (Center for Vascular Biology, University of Connecticut Health Center, Farmington, CT) for helpful comments and discussion, Dr. John A. Watts (Carolinas Medical Center, Charlotte, NC) for suggestions, and Dr. Rixin Wang (Yale Center for Medical Informatics, New Haven, CT) for statistical assistance in the data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, J., Shan, Y., Lambrecht, R.W. et al. Differential regulation of human ALAS1 mRNA and protein levels by heme and cobalt protoporphyrin. Mol Cell Biochem 319, 153–161 (2008). https://doi.org/10.1007/s11010-008-9888-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9888-0