Abstract
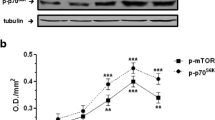
Insulin promotes protein accretion in cardiac and skeletal muscles through a stimulation of the mRNA translation initiation phase of protein synthesis. The present set of experiments examined the regulatory TSC2 signaling pathway that potentially contributes to the myocardial responsiveness of protein synthesis to insulin in post-absorptive male Sprague-Dawley rats in vivo. Heart and skeletal muscles were sampled from rats up to 1 h following intravenous injection of various doses of insulin. In cardiac muscle, TSC2 phosphorylation was elevated only at the highest plasma insulin concentration (386 ng/ml). In contrast, the extent of mTOR phosphorylation either on Ser(2448) or Ser(2481) was raised at 24-fold less concentration of insulin and corresponded with increased phosphorylation of PKB(Thr308) or PKB(Ser473). In gastrocnemius, TSC2 phosphorylation was elevated at plasma insulin concentrations (16 ng/ml) lower than that observed in cardiac muscle (386 ng insulin/ml). The increased TSC2 phosphorylation corresponded with a marked stimulation of PKB phosphorylation. However, mTOR(Ser2448) or mTOR(Ser2481) phosphorylation was not elevated until the plasma insulin concentration reached 97 ng/ml. The results indicate there is a dissociation of TSC2 and mTOR phosphorylation in vivo.












Similar content being viewed by others
References
Balage M, Sinaud S, Prod’Homme M et al (2001) Amino acids and insulin are both required to regulate assembly of eIF4E·eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab 281:E565–E574
Anthony JC, Lang CH, Crozier SJ et al (2002) Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab 282:E1092–E1101
Tee AR, Fingar DC, Manning BD et al (2002) Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 99:13571–13576. doi:10.1073/pnas.202476899
van Slegtenhorst M, Nellist M, Nagelkerken B et al (1998) Interaction between humartrin and tuberlin, the TSC1 and TSC2 gene products. Hum Mol Genet 7:1053–1057. doi:10.1093/hmg/7.6.1053
Garami A, Zwartkruis FJT, Nobukuni T et al (2003) Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and TSC2. Mol Cell 11:1457–1466. doi:10.1016/S1097-2765(03)00220-X
Inoki K, Li Y, Zhu T et al (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657. doi:10.1038/ncb839
Zhang Y, Gao X, Saucedo L et al (2003) Rheb is a direct target of the tuberous sclerosis tumor supressor proteins. Nat Cell Biol 5:578–581. doi:10.1038/ncb999
Dan HC, Sun M, Yang L et al (2002) Phosphotidylinositol 3-kinase/AKT pathway regulates tuberous sclerosis tumor supressor complex by phosphorylation of tuberin. J Biol Chem 277:35364–35370. doi:10.1074/jbc.M205838200
Manning BD, Tee AR, Logsdon MN et al (2002) Identification of the tuberous sclerosis complex-2 tumor supressor gene product tuberlin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell 10:151–162. doi:10.1016/S1097-2765(02)00568-3
Vary TC, Lang CH (2005) IGF-I activates the eIF4F system in cardiac muscle in vivo. Mol Cell Biochem 272:209–220. doi:10.1007/s11010-005-7551-6
Vary TC, Lynch CJ (2006) Meal feeding stimulates phosphorylation of multiple effector proteins regulating protein synthetic processes in rat hearts. J Nutr 136:2284–2290
Vary TC, Lynch CJ (2006) Meal feeding enhances formation of eIF4F in skeletal muscle: role of increased eIF4E availability and eIF4G phosphorylation. Am J Physiol Endocrinol Metab 290:E631–E642. doi:10.1152/ajpendo.00460.2005
Jefferson LS, Vary TC, Kimball SR (2001) Regulation of protein metabolism in muscle. In: Jefferson LS, Cherrington AD (eds) Handbook of physiology. Oxford University Press, New York, pp 529–552
Brown EJ, Beal PA, Keith CT et al (1995) Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature 377:441–446. doi:10.1038/377441a0
Raught B, Gingas A-C, Sonenberg N (2001) The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA 98:7037–7044. doi:10.1073/pnas.121145898
Sekulic A, Hudson CC, Homme JL et al (2000) A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 60:3504–3513
Peterson RT, Beal PA, Comb MJ et al (2000) FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275:7416–7423. doi:10.1074/jbc.275.10.7416
Vary TC, Lynch CJ (2005) Nutrient signaling to muscle and adipose tissue by leucine. In: Zempleni J, Dakshinamirti K (eds) Nutrient and cell signaling. CRC Press, Boca Raton, FL, pp 299–352
Vary TC, Lynch CJ (2007) Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr 137:1835–1843
Vary TC, Deiter G, Lynch CJ (2007) Rapamycin limits formation of active eukaryotic initiation factor 4F complex following meal feeding in rat hearts. J Nutr 137:1857–1862
Bolster DR, Vary TC, Kimball SR et al (2004) Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 134:1704–1710
Nave BT, Owens M, Withers DJ et al (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344:427–431. doi:10.1042/0264-6021:3440427
Avruch J (1998) Insulin signal transduction through protein kinase cascades. Mol Cell Biochem 182:31–42. doi:10.1023/A:1006823109415
Potter CJ, Pedrazza LG, Xu T (2002) AKT regulates growth by directly phosphorylating TSC2. Nat Cell Biol 4:658–662. doi:10.1038/ncb840
Vary TC, Deiter G, Lantry R (2007) Chronic alcohol feeding impairs mTOR(Ser2448) phosphorylation in rat hearts. Alcohol Clin Exp Res 31:43–51. doi:10.1111/j.1530-0277.2006.00285.x
Acknowledgments
This work was supported in part by National Institute on Alcohol Abuse and Alcoholism grant AA-12814 and National Institute General Medical Sciences GM-39277. We thank Dr. Christopher Lynch at Penn State University College of Medicine for kindly assessing plasma insulin concentrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsyth, S., Vary, T.C. Partial dissociation of TSC2 and mTOR phosphorylation in cardiac and skeletal muscle of rats in vivo. Mol Cell Biochem 319, 141–151 (2008). https://doi.org/10.1007/s11010-008-9887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9887-1