Abstract
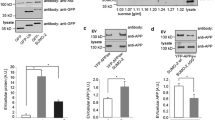
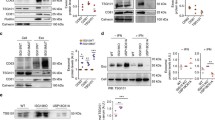
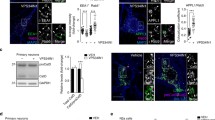
Neprilysin 2 (NEP2) has been recently identified as a new member of the M13 subfamily of zinc-dependent metalloproteases and shares a highly homologous amino acid sequence with neprilysin (EC 3.4.24.11, NEP). NEP2 has been reported to exist as membrane-bound and soluble secreted variants. To investigate mechanisms of regulating NEP2 activity, we developed a simple and sensitive method for measuring NEP2 activity using synthetic substrates with a fluorescent probe. NEP2 only cleaved Suc-Ala-Ala-Phe-AMC, while NEP cleaved both Dansyl-d-Ala-Gly-p-nitro-Phe-Gly and Suc-Ala-Ala-Phe-AMC. Using HEK293 cells stably expressing mouse NEP2, we evaluated the effects of various reagents affecting post-translational modification and protein trafficking on extracellular NEP2 activity secreted into the culture medium. Inhibition of N-glycosylation by tunicamycin reduced both the enzymatic activity of extracellular NEP2 and the molecular size of intracellular NEP2. Disruption of the Golgi apparatus with brefeldin A markedly reduced extracellular NEP2 activity in parallel with intracellular NEP2 protein level in HEK293 cells. In contrast, the cytoskeleton disrupting reagents, nocodazole and cytochalasin B barely affected NEP2 activity. Two distinct calcium-perturbing reagents, a calcium ionophore A23187 and thapsigargin, reduced extracellular NEP2 activity. However, A23187-mediated down-regulation was not rescued by co-treatment with inhibitors of MAPK, calmodulin, or the proteasome/calpains. In conclusion, we established a simple and sensitive protocol which was able to discriminate NEP2 and NEP activity, and showed that intracellular transport and secretion of NEP2 is regulated by processes such as glycosylation, ER-Golgi transport, and intracellular calcium levels.






Similar content being viewed by others
Abbreviations
- NEP:
-
Neprilysin
- NEP2:
-
Neprilysin 2
- HPLC:
-
High-performance liquid chromatography
- HEK293 cells:
-
Human embryonic kidney 293 cells
- MAPK:
-
Mitogen-activated protein kinase
References
Ikeda K, Emoto N, Raharjo SB et al (1999) Molecular identification and characterization of novel membrane-bound metalloprotease, the soluble secreted form of which hydrolyzes a variety of vasoactive peptides. J Biol Chem 274:32469–32477
Ghaddar G, Ruchon AF, Carpentier M et al (2000) Molecular cloning and biochemical characterization of a new mouse testis soluble-zinc-metallopeptidase of the neprilysin family. Biochem J 347:419–429
Malfroy B, Schofield PR, Kuang WJ et al (1987) Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun 144:59–66
Shimada K, Takahashi M, Tanzawa K (1994) Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem 269:18275–18278
Emoto N, Yanagisawa M (1995) Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem 270:15262–15268
Lee S, Zambas ED, Marsh WL, Redman CM (1991) Molecular cloning and primary structure of Kell blood group protein. Proc Natl Acad Sci USA 88:6353–6357
Du L, Desbarats M, Viel J et al (1996) cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics 36:22–28
Kiryu-Seo S, Sasaki M, Yokohama H et al (2000) Damage-induced neuronal endopeptidase (DINE) is a unique metallopeptidase expressed in response to neuronal damage and activates superoxide scavengers. Proc Natl Acad Sci USA 97:4345–4350
Iwata N, Tsubuki S, Takaki Y et al (2000) Identification of the major Aβ1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 6:143–150
Turner AJ, Isaac RE, Coates D (2001) The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23:261–269
Rose C, Voisin S, Gros C et al (2002) Cell-specific activity of neprilysin 2 isoforms and enzymic specificity compared with neprilysin. Biochem J 363:697–705
Ouimet T, Facchinetti P, Rose C et al (2000) Neprilysin II: A putative novel metalloprotease and its isoforms in CNS and testis. Biochem Biophys Res Commun 271:565–570
Oh-hashi K, Nagai T, Tanaka T et al (2005) Determination of hypoxic effect on neprilysin activity in human neuroblastoma SH-SY5Y cells using a novel HPLC method. Biochem Biophys Res Commun 334:380–385
Florentin D, Sassi A, Roques BP (1984) A highly sensitive fluorometric assay for “enkephalinase,” a neutral metalloendopeptidase that releases tyrosine-glycine-glycine from enkephalins. Anal Biochem 141:62–69
Mumford RA, Strauss AW, Powers JC et al (1980) A zinc metalloendopeptidase associated with dog pancreatic membranes. J Biol Chem 255:2227–2230
Hara H, Oh-hashi K, Yoneda S et al (2006) Elevated neprilysin activity in vitreous of patients with proliferative diabetic retinopathy. Mol Vis 12:977–982
da Costa SR, Yarber FA, Zhang L et al (1998) Microtubules facilitate the stimulated secretion of β-hexosaminidase in lacrimal acinar cells. J Cell Sci 111:1267–1276
Santell L, Marotti K, Bartfeld NS et al (1992) Disruption of microtubules inhibits the stimulation of tissue plasminogen activator expression and promotes plasminogen activator inhibitor type 1 expression in human endothelial cells. Exp Cell Res 201:358–365
Hay JC (2007) Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep 8:236–240
Carreno FR, Goni CN, Castro LM, Ferro ES (2005) 14-3-3 epsilon modulates the stimulated secretion of endopeptidase 24.15. J Neurochem 93:10–25
Lenz W, Herten M, Gerzer R, Drummer C (1999) Regulation of natriuretic peptide (urodilatin) release in a human kidney cell line. Kidney Int 55:91–99
Kim MS, Lim WK, Park RK et al (2005) Involvement of mitogen-activated protein kinase and NF-kB activation in Ca2+-induced IL-8 production in human mast cells. Cytokine 32:226–233
Menconi MJ, Wei W, Yang H et al (2004) Treatment of cultured myotubes with the calcium ionophore A23187 increases proteasome activity via a CaMK II-caspase-calpain-dependent mechanism. Surgery 136:135–142
Sharma AK, Rohrer B (2004) Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem 279:35564–35572
Yatsu T, Kurosawa H, Hayashi M, Satoh S (2003) The role of Ca2+ in the control of renin release from dog renal cortical slices. Eur J Pharmacol 458:191–196
Ribeiro CM, McKay RR, Hosoki E et al (2000) Effects of elevated cytoplasmic calcium and protein kinase C on endoplasmic reticulum structure and function in HEK293 cells. Cell Calcium 27:175–185
Kang T, Nagase H, Pei D (2002) Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network. Cancer Res 62:675–681
Vey M, Schäfer W, Berghöfer S et al (1994) Maturation of the trans-Golgi network protease furin: compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. J Cell Biol 127:1829–1842
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Okada T, Yoshida H, Akazawa R et al (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594
Shirotani K, Tsubuki S, Iwata N et al (2001) Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem 276:21895–21901
Roques BP, Fournie-Zaluski MC, Soroca E et al (1980) The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature 288:286–288
Baumer P, Danquechin Dorval E et al (1992) Effects of acetorphan, an enkephalinase inhibitor, on experimental and acute diarrhoea. Gut 33:753–758
Bralet J, Schwartz JC (2001) Vasopeptidase inhibitors: an emerging class of cardiovascular drugs. Trends Pharmacol Sci 22:106–109
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh-hashi, K., Ohkubo, K., Shizu, K. et al. Biosynthesis, processing, trafficking, and enzymatic activity of mouse neprilysin 2. Mol Cell Biochem 313, 103–111 (2008). https://doi.org/10.1007/s11010-008-9747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9747-z