Abstract
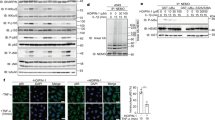
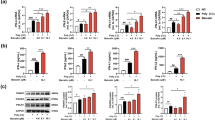
Alloferon is a 13-amino acid peptide isolated from the bacteria-challenged larvae of the blow fly Calliphora vicina. The pharmaceutical value of the peptide has been well demonstrated by its capacity to stimulate NK cytotoxic activity and interferon (IFN) synthesis in animal and human models, as well as to enhance antiviral and antitumor activities in mice. Antiviral and the immunomodulatory effectiveness of alloferon have also been supported clinically proved in patients suffering with herpes simplex virus (HSV) and human papilloma virus (HPV) infections. To elucidate molecular response to alloferon treatment, we initially screened a model cell line in which alloferon enhanced IFN synthesis upon viral infection. Among the cell lines tested, Namalva was chosen for further proteomic analysis. Fluorescence difference gel electrophoresis (DIGE) revealed that the levels of a series of antioxidant proteins decreased after alloferon treatment, while at least three glycolytic enzymes and four heat-shock proteins were increased in their expression levels. Based on the result of our proteomic analysis, we speculated that alloferon may activate the NF-κB signaling pathway. IκB kinase (IKK) assay, Western blot analysis on IκBα and its phosphorylated form at Ser 32, and an NF-κB reporter assay verified our proteomics-driven hypothesis. Thus, our results suggest that alloferon potentiates immune cells by activating the NF-κB signaling pathway through regulation of redox potential. Since NF-κB activation is involved in IFN synthesis, our results provide further clues as to how the alloferon peptide may stimulate IFN synthesis.




Similar content being viewed by others
Change history
19 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11010-022-04573-3
Abbreviations
- DIGE:
-
Fluorescence difference gel electrophoresis
- GSTP1:
-
Glutathione S-transferase P
- HPV:
-
Human papilloma virus
- HSV:
-
Herpes simplex virus
- IDPc:
-
NADP+-dependent isocitrate dehydrogenase
- IFN:
-
Interferon
- LC-ESI-MS/MS:
-
Liquid chromatography-electrospray ionization-tandem mass spectrometry
- MALDI-MS/MS:
-
Matrix-assisted laser desorption ionization tandem mass spectrometry
- PDIA3:
-
Protein disulfide-isomerase A3
- PI3K:
-
Phosphatidylinositol-4, 5-bisphosphate 3-kinase
- PRX4:
-
Peroxiredoxin 4
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptor
- TR1:
-
Thioredoxin reductase 1
References
Bulet P, Hetru C, Dimarcq JL et al (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23:329–344
Chernysh S, Kim SI, Bekker G et al (2002) Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA 99:12628–12632
Chernysh SI, Filatova NA, Chernysh NS et al (2004) Cytotoxic activity of blowfly Calliphora vicina hemocytes. J Insect Physiol 50:777–781
Ershov FI, Kubanova AA, Pinegin BV et al (2003) Allokine-α effect on the course of chronic genital herpes relapses. Materia Medica 40:103–111
Safronnikova NR, Chernysh SI (2006) Novelty in the treatment of papilloma virus infections: therapeutic properties of alloferons. In: Merabishvily VM, Safronnikova NR (eds) Prevention of virus-dependent oncological diseases. Research Institute of Oncology, St. Petersburg, Russia
Pandey A, Mann M (2000) Proteomics to study genes and genomes. Nature 405:837–846
Wang Y, Chiu JF, He QY (2006) Proteomics approach to illustrate drug action mechanisms. Curr Drug Discov Technol 3:199–209
Sidorova MV, Molokoedov AS, Kudriavtseva EV et al (2006) The synthesis of immunomodulating peptide alloferon, the active component of the antiviral drug allokine-α. Bioorg Khim 32:151–160
Fernandez RC, Lee SH, Fernandez LA et al (1986) Production of interferon by peripheral blood mononuclear cells from normal individuals and patients with chronic lymphocytic leukemia. J Interferon Res 6:573–580
Ryu M-J, Kim D, Kang U-B et al (2007) Proteomic analysis of γ-butyrolactone-treated mouse thalamus reveals dysregulated proteins upon absence seizure. J Neurochem 102:646–656
Figeys D, Gygi SP, Zhang Y et al (1998) Electrophoresis combined with novel mass spectrometry techniques: powerful tools for the analysis of proteins and proteomes. Electrophoresis 19:1811–1818
McCormack AL, Schieltz DM, Goode B et al (1997) Direct analysis and identification of proteins in mixtures by LC/MS/MS and database searching at the low-femtomole level. Anal Chem 69:767–776
Droin N, Beauchemin M, Solary E et al (2000) Identification of a caspase-2 isoform that behaves as an endogenous inhibitor of the caspase cascade. Cancer Res 60:7039–7047
Chen W, Wang J, An H et al (2005) Heat shock up-regulates TLR9 expression in human B cells through activation of ERK and NF-κB signal pathways. Immunol Lett 98:153–159
Gloire G, Legrand-Poels S, Piette J (2006) NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Kabe Y, Ando K, Hirao S et al (2005) Redox regulation of NF-κB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 7:395–403
Hayden MS, West AP, Ghosh S (2006) NF-κB and the immune response. Oncogene 25:6758–6780
EntoPharm Co., Ltd.: ALLOFERON: first immunomodulatory agent from insect. http://www.entopharm.com/eng/alloferon.htm
Unlu M, Morgan ME, Minden JS (1997) Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18:2071–2077
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Fox CJ, Hammerman PS, Thompson CB (2005) Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5:844–852
Greiner EF, Guppy M, Brand K (1994) Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem 269:31484–31490
Frauwirth KA, Riley JL, Harris MH et al (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769–777
Doughty CA, Bleiman BF, Wagner DJ et al (2006) Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 107:4458–4465
Jin DY, Chae HZ, Rhee SG et al (1997) Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J Biol Chem 272:30952–30961
Higuchi T, Watanabe Y, Waga I (2004) Protein disulfide isomerase suppresses the transcriptional activity of NF-κB. Biochem Biophys Res Commun 318:46–52
Lee SM, Koh HJ, Park DC et al (2002) Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32:1185–1196
Bauskin AR, Alkalay I, Ben-Neriah Y (1991) Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell 66:685–696
Brumell JH, Burkhardt AL, Bolen JB et al (1996) Endogenous reactive oxygen intermediates activate tyrosine kinases in human neutrophils. J Biol Chem 271:1455–1461
Ding J, Takano T, Gao S et al (2000) Syk is required for the activation of Akt survival pathway in B cells exposed to oxidative stress. J Biol Chem 275:30873–30877
Lee SR, Kwon KS, Kim SR et al (1998) Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273:15366–15372
Meng TC, Fukada T, Tonks NK (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9:387–399
Singh DK, Kumar D, Siddiqui Z et al (2005) The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121:281–293
Hehner SP, Breitkreutz R, Shubinsky G et al (2000) Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol 165:4319–4328
Williams MS, Kwon J (2004) T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med 37:1144–1151
Sulciner DJ, Irani K, Yu ZX et al (1996) rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol Cell Biol 16:7115–7121
Storz P, Toker A (2003) Protein kinase D mediates a stress-induced NF-κB activation and survival pathway. EMBO J 22:109–120
Luka J, Lindahl T, Klein G (1978) Purification of the Epstein–Barr virus-determined nuclear antigen from Epstein–Barr virus-transformed human lymphoid cell lines. J Virol 27:604–611
Bridgen PJ, Anfinsen CB, Corley L et al (1977) Human lymphoblastoid interferon. Large scale production and partial purification. J Biol Chem 252:6585–6587
Grandvaux N, ten Oever BR, Servant MJ et al (2002) The interferon antiviral response: from viral invasion to evasion. Curr Opin Infect Dis 15:259–267
Acknowledgements
We thank Profs. Wang-Jae Lee and Jae Seung Kang (Seoul National University College of Medicine, Seoul, Korea) for their critical review of the manuscript. This study was financially supported by the Korea-Russia Scientific and Technological Cooperation Center, affiliated at Korea Institute of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, MJ., Anikin, V., Hong, SH. et al. Activation of NF-κB by alloferon through down-regulation of antioxidant proteins and IκBα. Mol Cell Biochem 313, 91–102 (2008). https://doi.org/10.1007/s11010-008-9746-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9746-0