Abstract
The synchronization of the circadian signals to external or suprachiasmatic nucleus stimulation in the peripheral clocks is essential for maintaining the usual function of human body. However, aging will disrupt the synchronization of peripheral circadian rhythms, thus leading to some age-associated diseases. Up to now, little is known about the modification of the oscillatory rhythms in aged cells. A recent report showed that cell senescence in vascular human smooth muscle cells (HSMCs) altered circadian rhythms by a dysregulation of rhythmic gene expression. Furthermore, this alteration could be reversed by telomerase reconstitution. To test whether telomerase reconstitution can restore disrupted circadian rhythm in other types of senescent cells, we used fibroblasts as cell models to profoundly investigate the relationship between cell senescence and circadian rhythm modulation. We found that the response of rhythmic gene expression to serum stimulation was markedly attenuated in senescent fibroblasts, telomerase-reconstituted fibroblasts reset the circadian oscillation of rhythmic gene expression, and the activation of pERK-CREB and p38-CREB pathways might be involved in the circadian rhythm resetting. These findings suggested that telomerase reconstitution might be a good way to reset synchronization of peripheral circadian rhythms disrupted in senescent tissues.




Similar content being viewed by others
Change history
25 June 2020
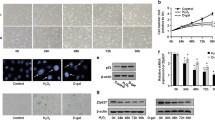
In the original article, Fig.��4b was published incorrectly in which four to five lanes in Pi-ERK and Pi-CREB panels look very similar to each other (Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts, Mol Cell Biochem, 2008, 313:11���18). Since this image was stored in The Experiment Center of the West China Second University Hospital, Sichuan University, which was dissoluted in 2012, the original data cannot be traced. Experiments were therefore redone to verify the result and the correct version of Fig.��4b is provided in this correction.
References
Halberg F (1983) Quo vadis basic and clinical chronobiology: promise for health maintenance. Am J Anat 168:543–594
Monk TH (2005) Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms 20:366–374
Hofman MA (2000) The human circadian clock and aging. Chronobiol Int 17:245–259
Hofman MA, Swaab DF (2006) Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 5:33–51
Yamazaki S, Straume M, Tei H et al (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA 99:10801–10806
Kolker DE, Fukuyama H, Huang DS et al (2003) Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms 18:159–169
Kunieda T, Minamino T, Katsuno T et al (2006) Cellular senescence impairs circadian expression of rhythmic genes in vitro and in vivo. Circ Res 98:532–539
Xie HQ, Yang ZM, Qu Y (2002) Culture of human fibroblasts transfected by human telomerase reverse transcriptase eukaryotic expression plasmid pGRN145 and their biological characteristics in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 16:195–199
Maier AB, Westendorp RJ, Heemst DV (2007) β-Galactosidase activity as a biomarker of replicative senescence during the course of human fibroblast cultures. Ann N Y Acad Sci 1100:323–332
Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93:929–937
Obrietan K, Impey S, Smith D et al (1999) Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem 274:17748–17756
Travnickova BZ, Cermakian N, Reppert SM et al (2002) Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99:7728–7733
Fukada Y, Okano T (2002) Circadian clock system in the pineal gland. Mol Neurobiol 25:19–30
Halberg F (1980) Chronobiology: methodological problems. Acta Med Romana 18:399–440
McNamara P, Seo SP, Rudic RD et al (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105:877–889
Nagoshi E, Saini C, Bauer C et al (2004) Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 19:693–705
Welsh DK, Yoo SH, Liu AC et al (2004) Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol 14:2289–2295
Akashi M, Nishida E (2000) Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev 14:645–649
Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290
King DP, Takahashi JS (2000) Molecular genetics of the circadian rhythms in mammals. Annu Rev Neurosci 23:712–742
Preitner N, Damiola F, Lopez-Molina L et al (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260
Allada R, Emery P, Takahashi JS et al (2001) Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24:1091–1119
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30570902 to Zhengrong Wang, Nos. 30570623 and 30770748 to Dezhi Mu) and Doctoral Program of Ministry of Education of China (No. 20050610094 to Yi Qu and No. 20070610092 to Dezhi Mu).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qu, Y., Mao, M., Li, X. et al. Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts. Mol Cell Biochem 313, 11–18 (2008). https://doi.org/10.1007/s11010-008-9736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9736-2