Abstract
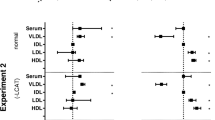
The plasma (P), VLDL (V) triglyceride and apoB (B) clearance rates were measured both as ‘mass’ clearance (k 1) and ‘within the particle’ clearance in three patient groups (E33, E23 and E34 phenotypes) at heparin-induced lipolysis in vivo. The lipid (C)- and apoE (E)-specific lipoprotein profiles both before and after heparin were followed by capillary isotachophoresis. The displacement of apoE by exogenous apoC-III at plasma titration in vitro was measured as well. The phenotype-sensitive lipoprotein networks were constructed based on an established set of metabolic rules. The k 1(V) values did not differ between the three groups, but the lower k 1(P) values showed significant differences. The k 1(P) values for E33 and E23 groups were twofold higher compared to E34. A twofold increase in the rate constant for VLDL triglyceride clearance within the particle in E34 group compared to E23 reflected the inhibition of lipolysis by apoE2. For E33 group, (i) the k 1(V) value was negatively correlated to the size of non-displaceable apoE pool in 2E lipoprotein and to the maximal apoE sorbtion capacity for 2E and 3E lipoproteins; (ii) the k 1(P) value was not associated to the apoE binding parameters; (iii) the k 1(V) value was positively correlated to the 4C level and the magnitude of apoC-III removal from VLDL particle; (iv) the k 1(P) value was positively correlated to the content of apoE, while negatively with apoC-III, in VLDL remnants. For E34 group, the k 1(V) value was positively correlated to 11C and 1–7C pool levels. Lipolysis- and receptor-mediated TG runways seem to be mostly balanced in E33 group, and VLDL TG clearance may be controlled by HDL through apoE dissociation from VLDLs and apolipoprotein accumulation within ‘fast’ HDLs at lipolysis.



Similar content being viewed by others
References
Rader DJ (2006) Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest 116:3090–3100. doi:10.1172/JCI30163
Kuusi T, Ehnholm C, Viikari J et al (1989) Postheparin plasma lipoprotein and hepatic lipase are determinants of hypo- and hyperalphalipoproteinemia. J Lipid Res 30:1117–1126
Austin MA (2000) Triglyceride, small, dense low-density lipoprotein, and the atherogenic lipoprotein phenotype. Curr Atheroscler Rep 2:200–207. doi:10.1007/s11883-000-0021-4
Clark AB, Quarfordt SH (1985) Apolipoprotein effects on the lipolysis of perfused triglyceride by heparin-immobilized milk lipase. J Biol Chem 260:4778–4783
Rensen PCN, van Berkel TJC (1996) Apolipoprotein E effectively inhibits lipoprotein lipase-mediated lipolysis of chylomicron-like triglyceride-rich lipid emulsions in vitro and in vivo. J Biol Chem 271:14791–14799. doi:10.1074/jbc.271.25.14791
Ehnholm C, Mahley RW, Chappell DA et al (1984) Role of apolipoprotein E in the lipolytic conversion of b-very low density lipoproteins to low density lipoproteins in type III hyperlipoproteinemia. Proc Natl Acad Sci USA 81:5566–5570. doi:10.1073/pnas.81.17.5566
Thuren T, Weisgraber KH, Sisson P et al (1992) Role of apolipoprotein E in hepatic lipase catalyzed hydrolysis of phospholipid in high-density lipoproteins. Biochemistry 31:2332–2338. doi:10.1021/bi00123a018
Dergunov AD, Shuvaev VV, Perova NV (1989) Topo-dynamic characteristics of human plasma VLDL apolipoproteins and efficiency of triacylglycerol hydrolysis by lipoprotein lipase. Biochim Biophys Acta 1005:79–86
Brown WV, Baginsky ML (1972) Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun 46:375–382. doi:10.1016/S0006-291X(72)80115-3
Landis BA, Rotolo FS, Meyers WC et al (1987) Influence of apolipoprotein E on soluble and heparin-immobilized hepatic lipase. Am J Physiol 252:G805–G810
Camacho D, de la Fuente A, Mendes P (2005) The origin of correlations in metabolomics data. Metabolomics 1:53–63. doi:10.1007/s11306-005-1107-3
Kose F, Weckwerth W, Linke T et al (2001) Visualizing plant metabolomic correlation networks using clique-metabolite matrices. Bioinformatics 17:1198–1208. doi:10.1093/bioinformatics/17.12.1198
Vance W, Arkin A, Ross J (2002) Determination of causal connectivities of species in reaction networks. Proc Natl Acad Sci USA 99:5816–5821. doi:10.1073/pnas.022049699
Hofmeyr JH, Kacser H, van der Merwe KJ (1986) Metabolic control analysis of moiety-conserved cycles. Eur J Biochem 155:631–641. doi:10.1111/j.1432-1033.1986.tb09534.x
Wang CS, Alaupovic P, Gregg RE et al (1987) Studies on the mechanism of hypertriglyceridemia in Tangier disease. Determination of plasma lipolytic activities, k1 values and apolipoprotein composition of the major lipoprotein density classes. Biochim Biophys Acta 920:9–19
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666. doi:10.1021/ja01318a036
Hofmeyr JH, Cornish-Bowden A (1996) Co-response analysis: a new experimental strategy for metabolic control analysis. J Theor Biol 182:371–380. doi:10.1006/jtbi.1996.0176
Forte TM, Krauss RM, Lindgren FT et al (1979) Changes in plasma lipoprotein distribution and formation of two unusual particles after heparin-induced lipolysis in hypertriglyceridemic subjects. Proc Natl Acad Sci USA 76:5934–5938. doi:10.1073/pnas.76.11.5934
Barrans A, Collet X, Barbaras R et al (1994) Hepatic lipase induces the formation of pre-β 1 high density lipoprotein (HDL) from triacylglycerol-rich HDL2. A study comparing liver perfusion to in vitro incubation with lipases. J Biol Chem 269:11572–11577
Chung BH, Dashti N (2000) Lipolytic remnants of human VLDL produced in vitro: effect of HDL levels in the lipolysis mixtures on the apoCs to apoE ratio and metabolic properties of VLDL core remnants. J Lipid Res 41:285–297
Dergunov AD, Smirnova EA, Merched A et al (2000) Structural peculiarities of the binding of very low density lipoproteins and low density lipoproteins to the LDL receptor in hypertriglyceridemia: role of apolipoprotein E. Biochim Biophys Acta 1484:29–40
Petersen M, Dyrby M, Toubro S et al (2005) Quantification of lipoprotein subclasses by proton nuclear magnetic resonance-based partial least-squares regression models. Clin Chem 51:1457–1461. doi:10.1373/clinchem.2004.046748
Murdoch SJ, Breckenridge WC (1995) Influence of lipoprotein lipase and hepatic lipase on the transformation of VLDL and HDL during lipolysis of VLDL. Atherosclerosis 118:193–212. doi:10.1016/0021-9150(95)05606-8
Murdoch SJ, Breckenridge WC (1996) Effect of lipid transfer proteins on lipoprotein lipase induced transformation of VLDL and HDL. Biochim Biophys Acta 1303:222–232
Aalto-Setala K, Fisher EA, Chen X et al (1992) Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest 90:1889–1900. doi:10.1172/JCI116066
Aalto-Setala K, Weinstock PH, Bisgaier CL et al (1996) Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoC-III transgenic mice. J Lipid Res 37:1802–1811
Rye K-A, Bright R, Psaltis M et al (2006) Regulation of reconstituted high density lipoprotein structure and remodeling by apolipoprotein E. J Lipid Res 47:1025–1036. doi:10.1194/jlr.M500525-JLR200
Dergunov AD, Novoselov AV, Visvikis S et al (2005) The composition, structural properties and binding of very-low-density and low-density lipoproteins to the LDL receptor in normo- and hypertriglyceridemia: relation to the apolipoprotein E phenotype. Biol Chem 386:441–452. doi:10.1515/BC.2005.053
Morton RE, Greene DJ (1994) Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J Lipid Res 35:836–847
Hime NJ, Drew KJ, Hahn C et al (2004) Apolipoprotein E enhances hepatic lipase-mediated hydrolysis of reconstituted high-density lipoprotein phospholipid and triacylglycerol in an isoform-dependent manner. Biochemistry 43:12306–12314. doi:10.1021/bi036305i
Fazio S, Marotti KR, Lee YL et al (1994) Co-expression of cholesteryl ester transfer protein and defective apolipoprotein E in transgenic mice alters plasma cholesterol distribution. Implications for the pathogenesis of type III hyperlipoproteinemia. J Biol Chem 269:32368–32372
Batal R, Tremblay M, Barrett PH et al (2000) Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res 41:706–718
Huang Y, Liu XQ, Rall SC Jr et al (1998) Apolipoprotein E2 reduces the low density lipoprotein level in transgenic mice by impairing lipoprotein lipase-mediated lipolysis of triglyceride-rich lipoproteins. J Biol Chem 273:17483–17490. doi:10.1074/jbc.273.28.17483
Zak Z, Lagrost L, Gautier T et al (2002) Expression of simian CETP in normolipidemic Fisher rats has a profound effect on large sized apoE-containing HDL. J Lipid Res 43:2164–2171. doi:10.1194/jlr.M200253-JLR200
Soderlund S, Soro-Paavonen A, Ehnholm C et al (2005) Hypertriglyceridemia is associated with preβ-HDL levels in subjects with familial low HDL. J Lipid Res 46:1643–1651. doi:10.1194/jlr.M400480-JLR200
Fielding CJ, Fielding PE (1995) Molecular physiology for reverse cholesterol transport. J Lipid Res 36:211–228
Dergunov AD, Hoy A, Smirnova EA et al (2003) Charge-based heterogeneity of human plasma lipoproteins at hypertriglyceridemia: capillary isotachophoresis study. Int J Biochem Cell Biol 35:530–543. doi:10.1016/S1357-2725(02)00359-X
Acknowledgements
A.D.D. thanks the Russian Foundation for Basic Research for financial support, grant 07-04-00377.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dergunov, A.D., Ponthieux, A., Mel’kin, M.V. et al. Capillary isotachophoresis study of lipoprotein network sensitive to apolipoprotein E phenotype. 2. ApoE and apoC-III relations in triglyceride clearance. Mol Cell Biochem 325, 25–40 (2009). https://doi.org/10.1007/s11010-008-0017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-0017-x