Abstract
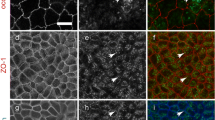
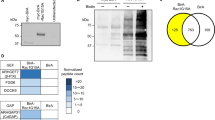
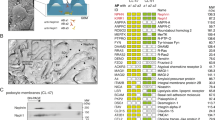
Densin is a member of LAP (leucine-rich repeat and PDZ domain) protein family that localizes in kidney to slit diaphragms, which are essential components of the glomerular filtration barrier. We have previously shown that densin interacts with a crucial slit diaphragm protein, nephrin. Here, we searched for novel binding partners of densin by yeast-two hybrid assay and identified beta-catenin. The interaction was confirmed by reciprocal co-immunoprecipitation assay and the binding site in densin was determined by GST-pull down assays. The GST-tagged densin was also able to pull down P-cadherin together with beta-catenin from human kidney glomerular lysates. Furthermore, densin co-localized with beta-catenin and F-actin in cell–cell contacts in cultured mouse podocytes. During cell–cell contact disruption and reformation densin and beta-catenin were dislocated from and relocated back to plasma membrane in a similar fashion. These and our previous findings suggest that densin may associate with the cadherin-catenin and nephrin complex(es), and may be involved in the formation of the cell–cell contacts including the slit diaphragm.





Similar content being viewed by others
Reference
Rodewald R, Karnovsky MJ (1974) Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60:423–433
Endlich K, Kriz W, Witzgall R (2001) Update in podocyte biology. Curr Opin Nephrol Hypertens 10:331–340
Schnabel E, Anderson JM, Farquhar MG (1990) The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111:1255–1263
Reiser J, Kriz W, Kretzler M, Mundel P (2000) The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11:1–8
Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Kihara I, Yamamoto T (2001) FAT is a component of glomerular slit diaphragms. Kidney Int 59:1003–1012
Ciani L, Patel A, Allen ND, Ffrench-Constant C (2003) Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 23:3575–3582
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR (2001) Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21:4829–4836
Sellin L, Huber TB, Gerke P, Quack I, Pavenstadt H, Walz G (2003) NEPH1 defines a novel family of podocin interacting proteins. Faseb J 17:115–117
Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, Walz G (2005) NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol 16:1693–1702
Ihalmo P, Palmen T, Ahola H, Valtonen E, Holthofer H (2003) Filtrin is a novel member of nephrin-like proteins. Biochem Biophys Res Commun 300:364–370
Apperson ML, Moon IS, Kennedy MB (1996) Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family. J Neurosci 16:6839–6852
Izawa I, Nishizawa M, Ohtakara K, Inagaki M (2002) Densin-180 interacts with delta-catenin/neural plakophilin-related armadillo repeat protein at synapses. J Biol Chem 277:5345–5350
Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB (2001) Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci 21:423–433
Robison AJ, Bartlett RK, Bass MA, Colbran RJ (2005) Differential modulation of Ca2+/calmodulin-dependent protein kinase ll activity by regulated interactions with NMDA receptor NR2B subunits and alpha -actinin. J Biol Chem 280:35329–35336
Ohtakara K, Nishizawa M, Izawa I, Hata Y, Matsushima S, Taki W, Inada H, Takai Y, Inagaki M (2002) Densin-180, a synaptic protein, links to PSD-95 through its direct interaction with MAGUIN-1. Genes Cells 7:1149–1160
Ahola H, Heikkila E, Astrom E, Inagaki M, Izawa I, Pavenstadt H, Kerjaschki D, Holthofer H (2003) A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol 14:1731–1737
Holthofer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D (1999) Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 155:1681–1687
Schiwek D, Endlich N, Holzman L, Holthofer H, Kriz W, Endlich K (2004) Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66:91–101
Gooding JM, Yap KL, Ikura M (2004) The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. Bioessays 26:497–511
Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW (2002) Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 161:1459–1466
Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR (2005) WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol 289:F431–F441
Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG (2004) Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 165:923–936
Hung AY, Sheng M (2002) PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702
Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275:73–77
Laura RP, Witt AS, Held HA, Gerstner R, Deshayes K, Koehler MF, Kosik KS, Sidhu SS, Lasky LA (2002) The Erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of delta-catenin and ARVCF. J Biol Chem 277:12906–12914
Gottardi CJ, Gumbiner BM (2001) Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol 11:R792–R794
Kobayashi N (2002) Mechanism of the process formation; podocytes vs. neurons. Microsc Res Tech 57:217–223
Bilder D, Perrimon N (2000) Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403:676–680
Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M (2000) LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol 2:415–422
Rangwala R, Banine F, Borg JP, Sherman LS (2005) Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem 280:11790–11797
Le TL, Yap AS, Stow JL (1999) Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol 146:219–232
Ivanov AI, Nusrat A, Parkos CA (2004) Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15:176–188
Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, Birnbaum D, Borg JP (2005) Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24:4330–4339
Reiser J, Pixley FJ, Hug A, Kriz W, Smoyer WE, Stanley ER, Mundel P (2000) Regulation of mouse podocyte process dynamics by protein tyrosine phosphatases rapid communication. Kidney Int 57:2035–2042
Caulfield JP, Reid JJ, Farquhar MG (1976) Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34:43–59
Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS (1995) Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA 92:8813–8817
Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ (1997) Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci 110:1013–1022
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Michaud JL, Lemieux LI, Dube M, Vanderhyden BC, Robertson SJ, Kennedy CR (2003) Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol 14:1200–1211
Acknowledgements
We are thankful to Claudia Kocksch for the skilled technical assistance and to Lauri Ylimaa for graphical work. Drs Masaki Inagaki and Ichiro Izawa are acknowledged for providing us with densin antibody and full-length human densin construct. This study was supported by the European Union (grants LSHB-CT-2003–503364, QLRT–2001 – 01215), Paavo Nurmi Foundation, the Academy of Finland (#213692), Finnish Diabetes Research foundation, The Sigrid Juselius Foundation and Finnish Heart Association.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Heikkilä, E., Ristola, M., Endlich, K. et al. Densin and beta-catenin form a complex and co-localize in cultured podocyte cell junctions. Mol Cell Biochem 305, 9–18 (2007). https://doi.org/10.1007/s11010-007-9522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9522-6