Abstract
Oxidative stress after traumatic brain injury may contribute to many of the pathophysiologic changes. Resveratrol, naturally present at high concentration in grape skin, seeds, and red wine, has significant antioxidant properties in a variety of in vitro and in vivo models. In this study, we investigate the effect of resveratrol on oxidative stress after traumatic brain injury in rat model.
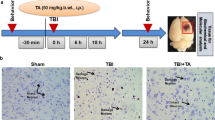
A total of 54 adult Wistar albino male rats weighing 250–300 g were used. The rats were allocated into three groups. The first group was control (sham-operated) group in which only a craniotomy was performed, the others were trauma and resveratrol groups. A 100 mg/kg single dose of resveratrol, freshly prepared by dissolving in 50% ethanol and diluted in physiological saline (2%), for resveratrol group, and 1 ml ethanol (2%) for trauma group, was administered intraperitoneally immediately after trauma. Weight-drop method was used for achieving head trauma. Then, all groups were separated into three subgroups for biochemical analysis, brain water content and histopathological assessment following trauma. Twenty-four hours after trauma brain water content and malondialdehyde (MDA), glutathione (GSH), nitric oxide (NO), xanthine oxidase (XO) levels of traumatic hemisphere were evaluated. Quantitative histopathological analysis was performed on 14th day postinjury. Trauma caused a significant increase in MDA, XO, NO levels and decrease in GSH level as compared to control group. Resveratrol administration significantly reduced MDA, XO and NO levels, increased GSH level, and also attenuated tissue lesion area. Our results indicate that treatment with resveratrol immediately after traumatic brain injury reduce oxidative stress and lesion volume. Future studies involving different doses and the dose–response relationship could promise better results.
Similar content being viewed by others
References
Yakovlev AG, Faden AI (2004) Mechanisms of neural cell death: implications for development of neuroprotective treatment strategies. NeuroRx 1:5–16
Hall ED, Braughler JM (1989) Central nervous system trauma and stroke. Physiological and pharmacological evidence for the involvement of oxygen radicals and lipid peroxidation. Free Rad Biol Med 6:303–313
Yukido I, Long DM (1990) The molecular basic of brain injury and brain edema. The role of oxygen free radicals. Neurosurgery 27:1–9
Awasthi D, Church DF, Torbati D, Carey ME, Pryor WA (1997) Oxidative stress following traumatic brain injury in rats. Surg Neurol 47:575–582
Anderson DK, Means ED (1985) Iron-induced lipid peroxidation in spinal cord: protection with mannitol and methylprednisolone. J Free Rad Biol Med 1:59–64
Demediuk P, Sanders RD, Clendenon NR, Means ED, Anderson DK, Horrocks LA (1985) Changes in lipid metabolism in traumatized spinal cord. Prog Brain Res 63:211–216
Soleas GJ, Diamandis EP, Goldberg DM (1997) Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal 11:287–313
Daniel O, Meier MS, Schlatter J, Frischhnecht P (1999) Selected phenolic compounds in cultivated plants: ecologic functions, health implications, and modulation by pesticides. Environ Health Perspect 107:109–114
Sobolev VS, Cole RJ (1999) Trans-resveratrol content in commercial peanuts and peanut products. J Agric Food Chem 47:1435–1439
Fremont L (2000) Biological effects of resveratrol. Life Sci 66:663–673
Inoue H, Umesono K, Nishimori T, Hirata Y, Tanabe T (1999) Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem Biophys Res Commun 254:292–298
Sinha K, Chaudhary G, Gupta YK (2002) Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci 6:655–665
Inoue H, Tanabe T, Umesono K (2000) Feedback control of cyclooygenase-2 gene expression through PPARgamma. J Biol Chem 275:28028–28032
Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK (2001) Resveratrol reduction of infarct size in Long_Evans rats subjected to focal cerebral ischemia. Life Sci 69:1057–1065
Bloomfield Rubins H, Davenport J, Babikian V, Brass LM, Collins D, Wexler L, Wagner S, Papademetriou V, Rutan G, Robins SJ (2001) VA-HIT Study Group: reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Circulation 103:2828–2833
Kimura Y, Okuda H, Arichi S (1985) Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim Biophys Acta 834:275–278
Yang YB, Piao YJ (2003) Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol Sin 24:703–710
Marklund N, Salci K, Lewen A, Hillered L (1997) Glycerol as a marker for post-traumatic membrane phospholipid degradation in rat brain. Neuroreport 717:109–117
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67–77
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Elman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Jungersten L, Edlund A, Petersson AS, Wennmalm A (1996) Plasma nitrate as an index of nitric oxide formation in man: analyses of kinetics and confounding factors. Clin Physiol 16:369–379
Zeballos GA, Bernstein RD, Thompson CI, Forfia PR, Seyedi N, Shen W, Kaminski PM, Wolin MS, Hintze TH (1995) Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation 91:2982–2988
Ozbek E, Turkoz Y, Gokdeniz R, Davarci M, Ozugurlu F (2000) Increased nitric oxide production in the spermatic vein of patients with varicocele. Eur Urol 37:172–175
Prajda N, Weber G (1975) Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett 59:245–249
Okiyama K, Smith DH, Gennarelli TA, Simon RP, Leach M, McIntosh TK (1995) The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J Neurochem 64:802–809
Huang L, Mehta MP, Nanda A, Zhang JH (2003) The role of multiple hyperbaric oxygenation in expanding therapeutic Windows after acute spinal cord injury in rats. J Neurosurg 99:198–205
Kontos HA, Povlishock JT (1986) Oxygen radicals in brain injury. Cent Nerv Syst Trauma 3:257–263
Kontos HA, Wei EP (1986) Superoxide production in experimental brain injury. J Neurosurg 64:803–807
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Nishio S, Yunoki M, Noguchi Y, Kawauchi M, Asari S, Ohmoto T (1997) Detection of lipid peroxidation and hydroxyl radicals in brain contusion of rats. Acta Neurochir Suppl (Wien) 70:84–86
Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S (2003) Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor α in mice. Neurosci Lett 352:203–206
Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK (2001) Resveratrol reduction of infarct size in Long_Evans rats subjected to focal cerebral ischemia. Life Sci 69:1057–1065
Ray PS, Maulik G, Cordis GA, Berteli AA, Berteli A, Das DK (1999) The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med 27:160–169
Olas B, Wachowicz B (2002) Resveratrol and vitamin C as antioxidants in blood platelets. Thrombosis Res 106:143–148
Chander V, Tirkey N, Chopra K (2005) Resveratrol, a polyphenolic phytoalexin protects against cyclosporine-induced nephrotoxicity through nitric oxide dependent mechanism. Toxicology 210:55–64
Tadolini B, Juliano C, Piu L, Franconi F, Cabrini L (2000) Resveratrol inhibition of lipid peroxidation. Free Radical Res 33:104–114
Chanvitayapongs S, Draczynska-Lusiak B, Sun AY (1997) Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport 8:1499–1502
Zini R, Morin C, Berteli A, Berteli AA, Tillement JP (1999) Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res 25:87–97
Mizutani K, Ikeda K, Kawai Y, Yamori Y (2001) Protective effective of resveratrol on oxidative damage in male and female stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 28:55–59
Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY (2002) Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 958:439–447
Hile R, Nishino T (1995) Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J 9:995–1003
Corte ED, Stirpe F (1972) The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J 126:739–745
McCord JM, Fridovich I (1968) The reduction of cytochrome C by milk xanthine oxidase. J Biol Chem 243:5753–5760
Lynch DR, Dawson TM (1994) Secondary mechanisms in neuronal trauma. Curr Opin Neurol 7:510–516
McIntosh TK (1994) Neurochemical sequelae of traumatic brain injury: therapeutic implications. Cerebrovasc Brain Metab Rev 6:109–162
Solaroglu I, Solaroglu A, Kaptanoglu E, dede S, Haberal A, Beskonakli E, Kilinc K (2003) Erythropoietin prevents ischemia-reperfusion from inducing oxidative damage in fetal rat brain. Childs Nerv Syst 19:19–22
Kanemitsu H, Tamura A, Kirino T, Oka H, Sano K, Iwamoto T (1989) Allopurinol inhibits uric acid accumulation in the rat brain following focal cerebral ischemia. Brain Res 499:367–370
Itoh T, Kawakami M, Yamauchi Y, Shimizu S, Nakamura M (1986) Effect of allopurinol on ischemia and reperfusion-induced cerebral injury in spontaneously hypertensive rats. Stroke 17:1284–1287
Solaroglu I, Okutan O, Kaptanoglu E, Beskonakli E, Kilinc K (2005) Increased xanthine oxidase activity after traumatic brain injury in rats. J Clin Neurosci 12:273–275
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
Peunova N, Enikolopov G (1993) Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature 364:450–453
Ikeda Y, Long DM (1990) The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 27:1–11
Eriskat J, Schurer L, Kempski O, Baetmann A (1994) Growth kinetics of primary brain tissue necrosis from a focal lesion. Acta Neurochir Suppl(Wien) 60:425–427
Plesnila N, Friedrich D, Eriskat J, Baethmann A, Stoffel M (2003) Relative cerebral blood flow during the secondary expansion of a cortical lesion rats. Neurosci Lett 345:85–88
Stoffel M, Blau C, Reinl H, Breidt J, Gersonde K, Baethmann A, Plesnila N (2004) Identification of brain tissue necrosis by MRI: validation by histomorphometry. J Neurotrauma 21:733–740
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented in the XIXth Annual Congress of Turkish Neurosurgery Society Antalya, Turkey, on May 27–31, 2005.
Rights and permissions
About this article
Cite this article
Ates, O., Cayli, S., Altinoz, E. et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 294, 137–144 (2007). https://doi.org/10.1007/s11010-006-9253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9253-0