Abstract
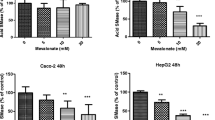
The influence of docosahexaenoic acid (DHA)- and eicosapentaenoic acid (EPA)-enriched phosphatidylcholine (PC) on the permeability, transport and uptake of phospholipids was evaluated in Caco-2 cells. The cells were grown on permeable polycarbonate transwell filters, thus allowing separate access to the apical and basolateral chambers. The monolayers of the cells were used to measure lucifer yellow permeability and transepithelial electrical resistance (TEER). Transcellular transportation of diphenylhexatriene (DPH) labeled-PC small unilamellar vesicles (SUV) from the apical to basolateral chamber, and uptake of the same SUV was monitored in the cell monolayers. Cell-membrane perturbation was evaluated to measure the release of lactate dehydrogenase and to determine the cell viability with sodium 2-(4-iodophenyl)-3-(4-nitrophenyl) -5-(2, 4-disulfophenyl)-2H-tetrazolium dye reduction assay. The lucifer yellow flux was 1.0 and 1.5 nmol/h/cm2 with 50 μM PC, and 17.0 and 23.0 nmol/h/cm2 with 100 μM PC when monolayers of Caco-2 cells were treated with DHA- and EPA-enriched PC, respectively. TEER decreased to 24 and 27% with 50 and 100 μM DHA-enriched PC, and to 25 and 30% with 50 and 100 μM EPA-enriched PC, respectively. Our results show that DHA- and EPA-enriched PC increases tight junction permeability across the Caco-2 cell monolayer whereas soy PC has no effect on tight junction permeability. Transportation and uptake of DHA- and EPA-enriched PC SUV differed significantly (P < 0.01) from those of soy PC SUV at all doses. We found that PC SUV transported across Caco-2 monolayer and was taken up by Caco-2 cells with very slight injury of the cell membrane up to 100 μM PC. Lactate dehydrogenase release and cell viability did not differ significantly between the treatment and control, emphasizing that injury was minimal. Our results suggest that DHA- and EPA-enriched PC enhance the permeability, transport and uptake of PC SUV across monolayers of Caco-2 cells. (Mol Cell Biochem xxx: 1–9, 2005)
Similar content being viewed by others
References
Perin N, Jarocka-Cyrta E, Keelan M, Clandinin T, Thomson A: Dietary lipid composition modifies intestinal morphology and nutrient transport in young rats. J Pediatr Gastroenterol Nutr 28: 46–53, 1999
Stillwell W, Ehringer W, Jenski LJ: Docosahexaenoic acid increases permeability of lipid vesicles and tumor cells. Lipids 28: 103–108, 1993
Roynette CE, Calder PC, Dupertuis YM, Pichard C: n-3 polyunsaturated fatty acids and colon cancer prevention. Clin Nutr 23: 139–151, 2004
de Deckere EA: Possible beneficial effect of fish and fish n-3 polyunsaturated fatty acids in breast and colorectal cancer. Eur J Cancer Prev 8: 213–221, 1999
Hollander D: Crohn's disease-a permeability disorder of the tight junction? Gut 26: 1621–1624, 1988
Hidalgo IJ, Raub TJ, Borchaedt RT: Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749, 1989
Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Sweilbaum A: Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47: 323–330, 1983
Vachon PH, Beaulieu JF: Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line, Gastroenterology 103: 414–423, 1992
Hauri HP, Sterchi EE, Bienz D, Fransen JAM, Marxer A: Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol 101: 838–851, 1985
Hidalgo IJ, Borchardt RT: Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta 1028: 25–30, 1990
Levy E, Mehran M, Seidman E: Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J 9: 626–635, 1995
Venkataram S, Awni WM, Jordan K, Rahman YE: Pharmacokinetics of two alternative dosage forms for cyclosporine: liposomes and intralipid. J Pharm Sci 9: 216–219, 1990
Schaffer JE, Lodish HF: Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79: 427–436, 1994
Tranchant T, Besson P, Honard C, Delane J, Antoine JM, Couet C, Gore J: Mechanisms and kinetics of alpha linolenic acid uptake in Caco-2 clone TC7. Biochim Biophys Acta 1345: 151–161, 1997
Bligh G, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959
Hossain Z, Konishi M, Hosokawa M, Takahashi K: Effect of polyunsaturated fatty acid-enriched phosphatidylcholine and phosphatidylserine on butyrate-induced growth inhibition, differentiation and apoptosis in Caco-2 cells. Cell Biochem Funct (in press), 2005
Lepage G, Roy CC: Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27: 114–120, 1986
Takahashi K, Hirano T, Saito M: Application of partition chromatographic theory for the analysis of marine triglyceride molecular species. Nippon Suisan Gakkaishi 54: 523–528, 1988
Jiang WG, Bryce RP, Horrobin DF, Mansel RE: Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem Biophys Res Commun 244: 414–420, 1998
Usami M, Muraki K, Iwamoto M, Ohata A, Matsushita E, Miki A: Effect of eicosapentaenoic (EPA) on tight junction permeability in intestinal monolayer cells. Clin Nutr 20: 351–359, 2001
Usami M, Komurasaki T, Hanada A, Kinoshita K, Ohata A: Effect of γ–linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition 19: 150–156, 2003
Roche HM, Terres AM, Black IB, Gibney MJ, Kelleher D: Fatty acids and epithelial permeability: effect of conjugated linoleic acid in Caco-2 cells. Gut 48: 797–802, 2001
Roig-Perez S, Guardiola F, Moreto M, Ferrer R: Lipid peroxidation induced by DHA enrichment modifies paracellular permeability in Caco-2 cells: protective role of taurine. J Lipid Res 45: 1418–1428, 2004
Wong V, Gumbiner BM: A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 136: 399–409, 1997
MacKay JA, Deen DF, Szoka Jr FC: Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res 1035: 139–153, 2005
Takeuchi H, Matsui Y, Yamamoto H, Kawashima Y: Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release 86: 235–242, 2003
Ho SY, Storch J: Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol 281: C1106-C1117, 2001
Wilson FA, Dietschy JM: The intestinal unstirred water layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta 363: 112–126, 1974
Lennernas H, Palm K, Fagerholm U, Artursson P: Correlation between paracellular and transcellular drug permeability in the human jejunum and Caco-2 monolayers. Int J Pharm 127: 1243–1251, 1996
Darimont C, Gradoux N, Cumin F, Baum HP, De Pover A: Differential regulation of intestinal and liver fatty acid-binding proteins in human intestinal cell line (Caco-2): role of collagen. Exp Cell Res 244: 441–447, 1998
Kamp F, Zakim D, Zhang F, Noy N, Hamilton JA: Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry 34: 11928–11937, 1995
Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA: Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation, Homology with CD36. J Biol Chem 268:17665–17668, 1993
Krishna G: Permeability of lipophilic compounds in drug discovery using in vitro human absorption model, Caco-2. Int J Pharm 222: 77–89, 2001
Frank O: Lecithin and Health: brain nutrients-phosphatidylcholine and serine, Vital Health Publishing, Bloomingdale, IL60108, 1998
Iwanaga K, Ono S, Narioka K, Kakemi M, Morimoto K, Yamashita S, Namba Y, Oku N: Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposomeè surface on the gi transit of insulin. J Pharm Sci 88: 248–252, 1999
Tanaka Y, Taki Y, Sakane T, Tanekazu N, Sezaki H, Yamashita H: Characterization of drug transport through tight-junctional pathway in Caco-2 monolayer: comparison with isolated rat jejunum and colon. Pharm Res 12: 523–528, 1995
Hashimoto K, Kawagishi H, Nakayama T, Shimizu M: Effect of capsianoside, a diterpene glycoside, on tight-junctional permeability. Biochim Biophys Acta 1323: 281–290 1997
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hossain, Z., Kurihara, H., Hosokawa, M. et al. Docosahexaenoic acid and eicosapentaenoic acid-enriched phosphatidylcholine liposomes enhance the permeability, transportation and uptake of phospholipids in Caco-2 cells. Mol Cell Biochem 285, 155–163 (2006). https://doi.org/10.1007/s11010-005-9074-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-9074-6