Abstract
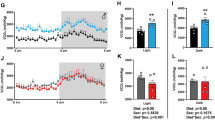
The circadian system is a flexible framework allowing a proper adjustment of physiological functions to the regularly changing environment. Pathways that are used to synchronize components of the circadian system have been shown to be susceptible to pathophysiological conditions. In our study, we investigated effects of streptozotocin (STZ)-induced diabetes mellitus on function of the circadian system at the level of melatonin synthesis and expression of per2 and dbp in the heart and liver in 8-week-old Wistar rats. Rhythmic pattern of clock gene per2 and transcription factor dbp in controls and STZ-treated animals was determined. Streptozotocin administration had a more substantial effect on per2 expression in the liver than in the heart. Pronounced phase advance in the rhythm of dbp expression in both the liver and the heart was observed. The melatonin rhythm reflecting the phase of the master clock was not affected by STZ application. Changes in per2 and dbp expression in the heart and liver imply alterations in input pathway or peripheral oscillators with possible consequences on function of analysed organs. (Mol Cell Biochem 270: 223–229, 2005)
Similar content being viewed by others
References
Akimoto Y, Kreppel LK, Hirano H, Hart GW: Localization of the O-linked N-acetylglucosamine transferase in rat pancreas. Diabetes 48: 2407–2413, 1999
Balsalobre A, Damiola F, Schibler U: A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937, 1998
Buijs RM, Kalsbeek A: Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2: 521–526, 2001
Champney TH, Holtorf AP, Craft CM, Reiter RJ: Hormonal modulation of pineal melatonin synthesis in rats and Syrian hamsters: Effects of streptozotocin-induced diabetes and insulin injections. Comp Biochem Physiol A 83: 391–395, 1986
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U: Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000
Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H: Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol 32: 985–996, 2000
Dhahbi JM, Mote PL, Cao SX, Spindler SR: Hepatic gene expression profiling of streptozotocin-induced diabetes. Diabetes Technol Ther 5: 411–420, 2003
Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC: Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol 12: 551–557, 2002
Fraser S, Cowen P, Franklin M, Franey C, Arendt J: Direct radioimmunoassay for melatonin in plasma. Clin Chem 29, 396–401, 1983
Hart GW, Kreppel LK, Comer FI, Arnold CS, Snow DM, Ye Z, Cheng X, DellaManna D, Caine DS, Earles BJ, Akimoto Y, Cole RN, Hayes BK: O-GlcNAcylation of key nuclear and cytoskeletal proteins: Reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology 6: 711–716, 1996
Klein DC: Photoneural regulation of the mammalian pineal gland. In: D. Evered, S. Clark (eds). Photoperiodism, Melatonin and the Pineal Gland, Vol. 117, Ciba Foundation Symposium, Pitman, London, 1985, pp 38–56
Klemfuss H, Clopton PL: Seeking tau: A comparison of six methods. J Interdisciplinary Cycle Res 24: 1–16, 1993
Konopka RJ, Benzer S: Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 68: 2112–2116, 1971
Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE: The potential mechanism of the diabetogenic action of streptozotocin: Inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase. Biochem J 356: 31–41, 2001
Kreppel LK, Blomberg MA, Hart GW: Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272: 9308–9315, 1997
Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C: Circadian expression of the steroid 15 a-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol 19: 6488–6499, 1999
Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U: The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J 16: 6762–6771, 1997
Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS: Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288: 483–492, 2000
McGarry JD: Lipid metabolism I: Utilisation and storage of energy in lipid form. In: T.M. Delvin (ed). Textbook of Biochemistry with Clinical Correlations. Wiley, New York, 2001, pp 693–726
Narayanan CS, Cui Y, Kumar A: DBP binds to the proximal promoter and regulates liver-specific expression of the human angiotensinogen gene. Biochem Biophys Res Commun 251: 388–393, 1998
Nelson W, Tong YL, Lee JK, Halberg F: Methods for cosinor-rhythmometry. Chronobiologia 6: 305–323, 1979
Oishi K, Kasamatsu M, Ishida N: Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun 317: 330–334, 2004
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB: Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002
Pando MP, Morse D, Cermakian N, Sassone-Corsi P: Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell 110: 107–117, 2002
Ralph MR, Foster RG, Davis FC, Menaker M: Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978, 1990
Ralph MR, Menaker M: A mutation of the circadian system in golden hamsters. Science 241: 1225–1227, 1988
Roos MD, Xie W, Su K, Clark JA, Yang X, Chin E, Paterson AJ, Kudlow JE: Streptozotocin, an analog of N-acetylglucosamine, blocks the removal of O-GlcNAc from intracellular proteins. Proc Assoc Am Physicians 110: 422–432, 1998
Rerup CC: Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22: 485–518, 1970
Rusak B, Zucker I: Neural regulation of circadian rhythms. Physiol Rev 59: 449–526, 1979
Silver R, LeSauter J, Tresco PA, Lehman MN: A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810–813, 1996
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM: Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019, 2000
Touitou Y, Haus E: Biologic Rhythms in Clinical and Laboratory Medicine. Springer-Verlag, Berlin, Heidelberg, New York, 1994, pp 1–708
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS: Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994
Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H: Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20: 4773–4781, 2000
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H: Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000
Young ME, Razeghi P, Taegtmeyer H: Clock genes in the heart: Characterization and attenuation with hypertrophy. Circ Res 88: 1142–1150, 2001
Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H: Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol 34: 223–231, 2002
Zeman M, Nosál’ová V, Bobek P, Zakálová M: Changes of endogenous melatonin and protective effect of diet containing pleuran and extract of black elder in colonic inflammation in rats. Biologia 56: 691–697, 2001
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herichová, I., Zeman, M., Stebelová, K. et al. Effect of streptozotocin-induced diabetes on daily expression of per2 and dbp in the heart and liver and melatonin rhythm in the pineal gland of Wistar rat. Mol Cell Biochem 270, 223–229 (2005). https://doi.org/10.1007/s11010-005-5323-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-5323-y