Abstract
Knocking out the regulatory β subunit of protein kinase CK2 in mice leads to early embryonic lethality. Heterozygous CK2β (CK2β+/−) knockout mice do not show an obvious phenotype. However, the number of heterozygous offsprings from CK2β+/− inter-crossings is lower than expected, meaning that some heterozygous embryos do not survive. Interestingly, CK2β+/− ES (Embryonic Stem) cells express a considerably lower level of CK2β than wild-type ES cells, whereas the level of CK2β in organs from heterozygous adult mice does not significantly differ from those of wild-type mice. The data suggest a compensatory mechanism that adjusts CK2β levels during development in the majority of, but not in all, cases (Mol Cell Biol {23:} 908–915, 2003).
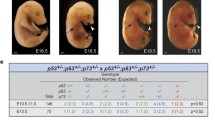
In order to find an explanation for the gene dosage effect observed for heterozygous offsprings, we analysed embryos at mid-gestation (E10.5) as well as wild-type and CK2β+/− ES cells for differences in growth rate and response to different stress agents. Analysis of E10.5 embryos generated from heterozygous matings revealed about 20% of smaller retarded CK2β+/− embryos. No correlation between CK2β levels in normal looking and retarded CK2β+/− embryos were found. However, a different post-translational form of CK2β protein has been detected in these retarded embryos. Cellular parameters such as growth rate and G1-, G2-checkpoints in ES cells were identical in both wild-type and CK2β+/− cells. When ES cells were injected to induce differentiated teratocarcinoma in syngenic mice, the size of the tumours correlated with the level of CK2β.
Similar content being viewed by others
References
Kusk M, Ahmed R, Thomsen B, Bendixen C, Issinger OG, Boldyreff B: Interaction of protein kinase CK2beta subunit within the holoenzyme and with other proteins. Mol Cell Biocehm 191: 51–58, 1999
Grein S, Raymond K, Cochet C, Pyerin W, Chambaz E, Filhol O: Searching interaction partners of protein kinase CK2beta subunit by two-hybrid screening. Mol Cell Biochem 191: 105–109, 1999
Chen M, Li D, Krebs EG, Cooper JA: The casein kinase II beta subunit binds to Mos and inhibits Mos activity. Mol Cell Biol 17: 1904–1912, 1997
Guerra B, Issinger OG, Wang JY: Modulation of human checkpoint kinase Chk1 by the regulatory beta-subunit of protein kinase CK2. Oncogene 22(32): 4933–4942, 2003
Bidwai AP, Reed JC, Glover CVC: Cloning and disruption of CKB1, the gene encoding the 38-kDA beta subunit of Saccharomyces cerevisiae casein kinase II (CKII). J Biol Chem 270: 10395–10404, 1995
Reed JC, Bidwai AP, Glover CVC: Cloning and disruption of CKB2, the gene encoding the 32-kDa regulatory beta-subunit of Saccharomyces cerevisiae casein kinase II. J Biol Chem 269: 18192–18200, 1994
Roussou I, Draetta G: The Schizosaccharomyces pombe casein kinase II alpha and beta subunits: Evolutionary conservation and positive role of the beta subunit. Mol Cell Biol 14: 576–586, 1994
Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J: Functional genomic analysis of C-elegans chromosome I by systemic RNA interference. Nature 408: 325–330, 2000
Jauch E, Melzig J, Brkulj M, Raabe T: In vivo functional analysis of Drosophila protein kinase casein kinase 2 (CK2) beta-subunit. Gene 298: 29–39, 2000
Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B: Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol 23: 908–915, 2003
Homma MK, Li D, Krebs EG, Yuasa Y, Homma Y: Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein. Proc Natl Acad Sci USA 99: 5959–5964, 2002
Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC: A structural basis for substrate specificities of protein Ser/Thr kinases: Primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol 16: 6486–6493, 1996
Sabapathy K, Klemm M, Jaenisch R, Wagner EF: Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J 16: 6217–6229, 1997
Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW: DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827, 2000
Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R: A role for cadherins in tissue formation. Development 122: 3185–3194, 1996
Martel V, Filhol O, Nueda A, Cochet C: Dynamic localization/association of protein kinase CK2 subunits in living cells: A role in its cellular regulation? Ann NY Acad Sci 973: 272–277, 2002
Meggio F, Pinna LA: One-thousand-and-one substrates of protein kinase CK2? FASEB J 17: 349–368, 2003
Muenstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG: Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem 189: 251–217, 1990
Gotz C, Scholtes P, Prowald A, Schuster N, Nastainczyk W, Montenarh M: Protein kinase CK2 interacts with a multi-protein binding domain of p53. Mol Cell Biochem 191: 111–120, 1999
Teitz T, Eli D, Penner M, Bakhanashvili M, Naiman T, Timme TL, Wood CM, Moses RE, Canaani D: Expression of the cDNA for the beta subunit of human casein kinase II confers partial UV resistance on xeroderma pigmentosum cells. Mutat Res 236: 85–97, 1990
Kato T, Delhase M, Hoffmann A, Karin M: CK2 is a terminal IkappaB kinase responsible for NF-kappaB activation during UV response. Mol Cell 12: 829–839, 2003
Romieu-Mourez R, Landesmann-Bollag E, Seldin DC, Sonenshein GE: Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cell. Cancer Res 62: 6770–6778, 2002
Toczyski DP, Galgoczy DJ, Hartwell LH: CDC5 and CKII control adaptation to the yeast DNA damage check point. Cell 90: 1097–1106, 1997
Stalter G, Siemer S, Becht E, Ziegler M, Remberger K, Issinger OG: Asymmetric expression of protein kinase CK2 subunits in human kidney tumors. Biochem Biophys Res Commun 202: 141–147, 1994
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blond, O., Jensen, H.H., Buchou, T. et al. Knocking out the regulatory beta subunit of protein kinase CK2 in mice: Gene dosage effects in ES cells and embryos. Mol Cell Biochem 274, 31–37 (2005). https://doi.org/10.1007/s11010-005-3117-x
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3117-x