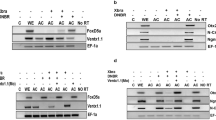
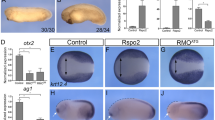
Abstract
CK2 is expressed widely in early embryonic development in several animal models, however its developmental role is unclear. One of the substrates of CK2 that is important in embryonic development is β-catenin, the transcriptional co-activator of the canonical Wnt signaling pathway. This pathway has been implicated in diverse aspects of embryonic development, including one of the earliest events in embryonic development, the establishment of the dorso-ventral embryonic axis. In Xenopus laevis, dorso-ventral axis formation is dependent upon stabilization of β-catenin in the future dorsal side of the embryo. Since CK2 phosphorylation of β-catenin stabilizes it, we hypothesized that CK2 might be critical to upregulation of β-catenin in Xenopus embryos and to the process of axis establishment. Our results demonstrate that CK2 is required for dorsal axis formation and is for normal upregulation of Wnt signaling genes and targets. Thus, CK2 is a regulator of endogenous axis formation in vertebrates.
Similar content being viewed by others
References
Prodon F, Pruliere G, Chenevert J, Sardet C: Establishment and expression of embryonic axes: comparisons between different model organisms. Med Sci (Paris) 20: 526–538, 2004
Weaver C, Kimelman D: Move it or lose it: axis specification in Xenopus. Development 131: 3491–3499, 2004
Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA: Microtubule-mediated transport of organelles and localization of beta- catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci USA 94: 1224–1229, 1997
Sokol SY: Wnt signaling and dorso-ventral axis specification in vertebrates. Curr Opin Genet Dev 9: 405–410, 1999
Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, {Kimelman} D, Moon RT: Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 136: 1123–1136, 1997
Dominguez I, Green JBA: Dorsal downregulation of GSK3(beta) by a non-Wnt-like mechanism is an early molecular consequence of cortical rotation in early Xenopus embryos. Development 127: 861–868, 2000
Song DH, Sussman DJ, Seldin DC: Endogenous protein kinase CK2 participates in Wnt Signaling in mammary epithelial cells. J Biol Chem 275: 23790–23797, 2000
Willert K, Brink M, Wodarz A, Varmus H, Nusse R: Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J 16: 3089–3096, 1997
Faust M, Montenarh M: Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res 301: 329–340, 2000
Schneider HR, Reichert GH, Issinger OG: Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur J Biochem 161: 733–738, 1986
Ahmed K, Davis AT, Wang H, Faust RA, Yu S, Tawfic S: Significance of protein kinase CK2 nuclear signaling in neoplasia. J Cell Biochem 79: 130–135, 2000
Jedlicki A, Hinrichs MV, Allende CC, Allende JE: The cDNAs coding for the alpha- and beta-subunits of Xenopus laevis casein kinase II. FEBS Lett 297: 280–284, 1992
Wilhelm V, Neckelman G, Allende JE, Allende CC: The genomic structure of two protein kinase CK2alpha genes of Xenopus laevis and features of the putative promoter region. Mol Cell Biochem 227: 175–183, 2001
Lieberman SL, Ruderman JV: CK2 beta, which inhibits Mos function, binds to a discrete domain in the N-terminus of Mos. Dev Biol 268: 271–279, 2004
Theis-Febvre N, Filhol O, Froment C, Cazales M, Cochet C, Monsarrat B, Ducommun B, Baldin V: Protein kinase CK2 regulates CDC25B phosphatase activity. Oncogene 22: 220–232, 2003
Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B: Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol 23: 908–915, 2003
Chen-Wu JL, Padmanabha R, Glover CV: Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol 8: 4981–4990, 1988
Padmanabha R, Chen-Wu JL, Hanna DE, Glover CV: Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol 10: 4089–4099, 1990
Xu X, Toselli PA, Russell LD, Seldin DC: Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet 23: 118–121, 1999
Dominguez I, Mizuno J, Wu H, Song DH, Symes K, Seldin DC: Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Biol 274: 110–124, 2004
Newport J, Kirschner M: A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30: 675–686, 1982
Nieuwkoop PD, Faber J: Normal Table of Xenopus Laevis. North-Holland Publishing Company, Amsterdam, 1967
Schneider VA, Mercola M: Spatially distinct head and heart inducers within the Xenopus organizer region. Curr Biol 9: 800–809, 1999
Wilhelm V, Rojas P, Gatica M, Allende CC, Allende JE: Expression of the subunits of protein kinase CK2 during oogenesis in Xenopus laevis. Eur J Biochem 232: 671–676, 1995
Sokol S, Christian JL, Moon RT, Melton DA: Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752, 1991
Smith WC, Harland RM: Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67: 753–765, 1991
Sokol SY, Klingensmith J, Perrimon N, Itoh K: Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121: 3487, 1995
Dominguez I, Itoh K, Sokol SY: Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA 92: 8498–8502, 1995
Pierce SB, Kimelman D: Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development 121: 755–765, 1995
He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB: Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374: 617–622, 1995
Guger KA, Gumbiner BM: Beta-catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol 172: 115–125, 1995
Cadigan KM, Nusse R: Wnt signaling: a common theme in animal development. Genes Dev 11: 3286–3305, 1997
Fagotto F, Guger K, Gumbiner BM: Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/beta-catenin signaling pathway, but not by Vg1, Activin or Noggin. Development 124: 453–460, 1997
Smalley MJ, Dale TC: Wnt signaling and mammary tumorigenesis. J Mammary Gland Biol Neoplasia 6: 37–52, 2001
Polakis P: Wnt signaling and cancer. Genes Dev 14: 1837–1851, 2000
Brown AM: Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res 3: 351–355, 2001
Giles RH, van Es JH, Clevers H: Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24, 2003
Seldin DC, Leder P: Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267: 894–897, 1995
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC: Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 20: 3247–3257, 2001
Song DH, Dominguez I, Mizuno J, Kaut M, Mohr SC, Seldin DC: CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem 278: 24018–24025, 2003
Vilk G, Saulnier RB, St Pierre R, Litchfield DW: Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J Biol Chem 274: 14406–14414, 1999
Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C: Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79: 791–803, 1994
Heasman J, Kofron M, Wylie C: Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol 222: 124–134, 2000
Hoppler S, Brown JD, Moon RT: Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10: 2805–2817, 1996
Sokol SY: Analysis of dishevelled signalling pathways during Xenopus development. Curr Biol 6: 1456–1467, 1996
Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D: GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93: 1031–1041, 1998
Kageura H: Activation of dorsal development by contact between the cortical dorsal determinant and the equatorial core cytoplasm in eggs of Xenopus laevis. Development 124: 1543–1551, 1997
Yang J, Tan C, Darken RS, Wilson PA, Klein PS: Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development 129: 5743–5752, 2002
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dominguez, I., Mizuno, J., Wu, H. et al. A role for CK2α/β in Xenopus early embryonic development. Mol Cell Biochem 274, 125–131 (2005). https://doi.org/10.1007/s11010-005-3073-5
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3073-5