Abstract
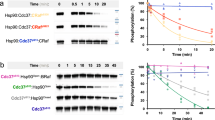
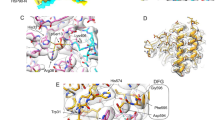
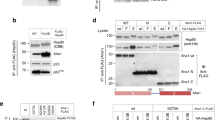
Protein kinase CK2 phosphorylates and regulates a large number of substrates but roles of CK2 in protein kinase-mediated signal transduction systems remain largely uncertain. Cdc37 is a protein kinase-targeting molecular chaperone and its function in cooperation with Hsp90 is required for various signaling kinases. In this article, interaction between CK2 and Cdc37 is described. We present evidence indicating that phosphorylation of Cdc37 by CK2 in conserved Ser13 in the N-terminal extremity was prerequisite for the efficient binding activity of Cdc37 to protein kinases including Akt, Cdk4, MOK, and Raf1. In addition, the phosphorylation of Cdc37 by CK2 was crucial for the recruitment of Hsp90 to the protein kinase-Cdc37 complexes. We observed that a subset of CK2 was associated with Hsp90 and Cdc37 in cells. Whereas Hsp90 and Cdc37 were exclusively distributed in the cytoplasm, CK2α and CK2β were localized mainly in the nucleus but also in the cytoplasm with different patterns. Moreover, direct association of Cdc37 with CK2α was observed in an E. coli system. Collectively, these findings indicated that a subpopulation of CK2 forms complexes with Hsp90 and Cdc37 in the cytoplasm and phosphorylates Cdc37, thus regulates the molecular chaperone activity of Cdc37. Since CK2 activity depends on Cdc37, CK2 and Cdc37 constitute a positive feedback machinery to control multiple Cdc37-dependent signaling protein kinases. The structure of Cdc37 and physiological importance of the CK2-Cdc37 interaction are discussed.
Similar content being viewed by others
References
Allende JE, Allende CC: Protein kinases. 4. Protein kinase CK2: an enzyme with multiple Substrates and a puzzling regulation. FASEB J 9: 313–323, 1995
Litchfield DW: Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369: 1–15, 2003
Pinna LA: The raison d’etre of constitutively active protein kinase: the lesson of CK2. Acc Chem Res 36: 378–384, 2003
Meggio F, Pinna LA: One-thousand-and-one substrates of protein kinase CK2? FASEB J 17: 349–368, 2003
Pinna LA: Protein kinase CK2: A challenge to canons. J Cell Sci 115: 3873–3878, 2002
Xu X, Landesman-Bollag E, Channavajhala PL, Seldin DC: Murine protein kinase CK2: Gene and oncogene. Mol Cell Biochem 191: 65–74, 1999
Seldin DC, Leder P: Casein kinase II a transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267: 894–897, 1995
Lindquist S, Craig EA: The heat shock proteins. Annu Rev Genet 22: 631–677, 1988
Welch WJ: Mammalian stress response: Cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiolog Rev 72: 1063–1081, 1992
Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G: The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther 79: 129–168, 1998
Yahara I, Minami Y, Miyata Y: The 90-kDa stress protein, Hsp90, is a novel molecular chaperone. Ann NY Acad Sci 851: 54–60, 1998
Pratt WB, Toft DO: Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228: 111–133, 2003
Buchner J: Hsp90 & Co: A holding for folding. Trends Biochem Sci 24: 136–141, 1999
Toft DO: Recent advances in the study of hsp90 structure and mechanism of action. Trends Endocrin Metabol 9: 238–243, 1998
Young JC, Moarefi I, Hartl FU: Hsp90: A specialized but essential protein-folding tool. J Cell Biol 154: 267–273, 2001
Hunter T, Poon RYC: Cdc37: A protein kinase chaperone? Trends Cell Biol 7: 157–161, 1997
MacLean M, Picard D: Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones 8: 114–119, 2003
Reed SI: The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95: 561–577, 1980
Gerber MR, Farrell A, Deshaies RJ, Herskowitz I, Morgan DO: Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA 92: 4651–4655, 1995
Cutforth T, Rubin GM: Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77: 1027–1036, 1994
Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J: Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 220–221, 2003
Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S: Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev 11: 1775–1785, 1997
Dey B, Lightbody JJ, Boschelli F: CDC37 is required for p60v-src activity in yeast. Mol Biol Cell 7: 1405–1417, 1996
Perdew GH, Wiegand H, Vanden Heuvel JP, Mitchell C, Singh SS: A 50 kilodalton protein associated with raf and pp60v-src protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry 36: 3600–3607, 1997
Miyata Y, Ikawa Y, Shibuya M, Nishida E: Specific association of a set of molecular chaperones including HSP90 and Cdc37 with MOK, a member of the MAP kinase superfamily. J Biol Chem 276: 21841–21848, 2001
Bandhakavi S, McCann RO, Hanna DE, Glover CVC: A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J Biol Chem 278: 2829–2836, 2003
Stepanova L, Leng X, Parker SB, Harper JW: Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev 10: 1491–1502, 1996
Miyata Y, Yahara I: The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem 267: 7042–7047, 1992
Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau M-C, Renoir J-M, Shirai R, Catelli M-G, Yahara I, Baulieu E-E: Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II (CK2): Regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci USA 94: 14500–14505, 1997
Shao J, Prince T, Hartson SD, Matts RL: Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J Biol Chem 278: 38117–38220, 2003
Miyata Y, Nishida E: CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone Cdc37. Mol Cell Biol 24: 4065–4074, 2004
Koyasu S, Nishida E, Kadowaki T, Matsuzaki F, Iida K, Harada F, Kasuga M, Sakai H, Yahara I: Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci USA 83: 8054–8058, 1986
Miyata Y, Yahara I: p53-independent association between SV40 large T antigen and the major cytosolic heat shock protein, HSP90. Oncogene 19: 1477–1484, 2000
Miyata Y, Yahara I: Interaction between casein kinase II and the 90-kDa stress protein, HSP90. Biochemistry 34: 8123–8129, 1995
Miyata Y, Akashi M, Nishida E: Molecular cloning and characterization of a novel member of the MAP kinase superfamily. Genes Cells 4: 299–309, 1999
Roe SM, Ali MM, Meyer P, Vaughan CK, Panaretou B, Piper PW, Prodromou C, Pearl LH: The mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 116: 87–98, 2004
Shao J, Grammatikakis N, Scroggins BT, Uma S, W H, Chen JJ, Hartson SD, Matts RL: Hsp90 regulates p50cdc37 function during the biogenesis of the active conformation of the heme-regulated eIF2 a kinase. J Biol Chem 276: 206–214, 2001
Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN, Cochran BH: p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol 19: 1661–1672, 1999
Shao J, Irwin A, Hartson SD, Matts RL: Functional dissection of cdc37: Characterization of domain structure and amino acid residues critical for protein kinase binding. Biochemistry 42: 12577–12588, 2003
Filhol O, Nueda A, Martel V, Gerber-Scokaert D, Benitez MJ, Souchier C, Saoudi Y, Cochet C: Live-cell fluorescence imaging reveals the dynamics of protein kinase CK2 individual subunits. Mol Cell Biol 23: 975–987, 2003
Fliss AE, Fang Y, Boschelli F, Caplan AJ: Differential in vivo regulation of steroid hormone receptor activation by Cdc37p. Mol Biol Cell 8: 2501–2509, 1997
Miyata Y, Nishida E: Supervision of multiple signaling protein kinases by the CK2-Cdc37 couple, a possible novel cancer therapeutic target. Ann NY Acad Sci 1030: 105–157, 2004
Maloney A, Workman P: HSP90 as a new therapeutic target for cancer therapy: The story unfolds. Expert Opin Biol Ther 2: 3–24, 2002
Miyata Y: Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr Pharm Des 11: 1131–1138, 2005
Isaacs JS, Xu W, Neckers L: Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 3: 213–217, 2003
Wang H, Yu S, Davis AT, Ahmed K: Cell cycle dependent regulation of protein kinase CK2 signaling to the nuclear matrix. J Cell Biochem 88: 812–822, 2003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyata, Y., Nishida, E. CK2 binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol Cell Biochem 274, 171–179 (2005). https://doi.org/10.1007/s11010-005-2949-8
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-2949-8