Abstract
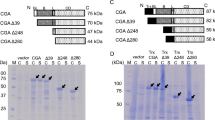
This study reports the revised and full-length cDNA sequence of bovine hexokinase type I obtained from bovine brain. Since dissimilarities have been observed between the published bovine hexokinase type I coding sequence (GenBank accession no. M65140) (Genomics 11: 1014-1024, 1991) and an analysed portion of bovine hexokinase type I gene, the entire open reading frame was re-sequenced and the ends of cDNA isolated by rapid amplification of cDNA ends. The coding sequences, when compared with the published bovine hexokinase type I, contained a large number of mismatches that lead to changes in the resulting amino acid sequence. The revisions result in a hexokinase type I cDNA of 3619 bp that encodes a protein of 917 amino acids highly homologous to human hexokinase type I. The expression of the recombinant full-length enzyme demonstrated that it was a catalytically active hexokinase. When characterised for its kinetic and regulatory properties, it displayed the same affinity for glucose and MgATP as the human hexokinase type I and was inhibited by glucose 6-phosphate competitively versus MgATP. The production of the N- and C-terminal recombinant halves of the enzyme followed by comparison with the full-length hexokinase indicated that the catalytic activity is located in the C-terminal domain. (Mol Cell Biochem 268: 9–18, 2005)
Similar content being viewed by others
References
Wilson JE: Hexokinases. Rev Physiol Biochem Pharmacol 126: 65–198, 1995
Tsai HJ, Wilson JE: Functional organization of mammalian hexokinases: Both N- and C-terminal halves of the rat type II isozyme possess catalytic sites. Arch Biochem Biophys 329: 17–23, 1996
Tsai HJ, Wilson JE: Functional organization of mammalian hexokinases: Characterization of chimeric hexokinases constructed from the N- and C-terminal domains of the rat type I and type II isozymes. Arch Biochem Biophys 316: 206–214, 1995
Tsai HJ, Wilson JE: Functional organization of mammalian hexokinases: Characterization of the rat type III isozyme and its chimeric forms, constructed with the N- and C-terminal halves of the type I and type II isozymes. Arch Biochem Biophys 338: 183–192, 1997
Gelb BD, Adams V, Jones SN, Griffin LD, MacGregor GR, McCabe ER: Targeting of hexokinase 1 to liver and hepatoma mitochondria. Proc Natl Acad Sci USA 89: 202–206, 1992
White TK, Wilson JE: Isolation and characterization of the discrete N- and C-terminal halves of rat brain hexokinase: retention of full catalytic activity in the isolated C-terminal half. Arch Biochem Biophys 274: 375–393, 1989
Bianchi M, Serafini G, Bartolucci E, Giammarini C, Magnani M: Enzymatic properties of overexpressed human hexokinase fragments. Mol Cell Biochem 189: 185–193, 1998
Fang TY, Alechina O, Aleshin AE, Fromm HJ, Honzatko RB: Identification of a phosphate regulatory site and a low affinity binding site for glucose 6-phosphate in the N-terminal half of human brain hexokinase. J Biol Chem 273: 19548–19553, 1998
Mulichak AM, Wilson JE, Padmanabhan K, Garavito RM: The structure of mammalian hexokinase-1. Nat Struct Biol 5: 555–560, 1998
Lazo PA, Sols A, Wilson JE: Brain hexokinase has two spatially discrete sites for binding of glucose-6-phosphate. J Biol Chem 255: 7548–7551, 1980
Aleshin AE, Fromm HJ, Honzatko RB: Multiple crystal forms of hexokinase I: new insights regarding conformational dynamics, subunit interactions, and membrane association. FEBS Lett 434: 42–46, 1998
Kabir F, Wilson JE: Mitochondrial hexokinase in brain of various species: differences in sensitivity to solubilization by glucose 6-phosphate. Arch Biochem Biophys 300: 641–650, 1993
White JA, Wilson JE: Structure of the gene for type I hexokinase from rat. Arch Biochem Biophys 343: 207–214, 1997
Ruzzo A, Andreoni F, Magnani M: Structure of the human hexokinase type I gene and nucleotide sequence of the 5′ flanking region. Biochem J 331: 607–613, 1998
Andreoni F, Ruzzo A, Magnani M: Structure of the 5′ region of the human hexokinase type I (HKI) gene and identification of an additional testis-specific HKI mRNA. Biochim Biophys Acta 1493: 19–26, 2000
Sebastian S, Edassery S, Wilson JE: The human gene for the type III isozyme of hexokinase: Structure, basal promoter, and evolution. Arch Biochem Biophys 395: 113–120, 2001
Griffin LD, Gelb BD, Wheeler DA, Davison D, Adams V, McCabe ER: Mammalian hexokinase 1: Evolutionary conservation and structure to function analysis. Genomics 11: 1014–1024, 1991
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467, 1977
Matz M, Shagin D, Bogdanova E, Britanova O, Lukyanov S, Diatchenko L, Chenchik A: Amplification of cDNA ends based on template-switching effect and step-out PCR. Nucleic Acids Res 27: 1558–1560, 1999
Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS: ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19: 4008, 1991
Bianchi M, Serafini G, Corsi D, Magnani M: High-level expression and purification of a human “mini”-hexokinase. Protein Expr Purif 7: 58–66, 1996
Bianchi M, Crinelli R, Serafini G, Giammarini C, Magnani M: Molecular bases of hexokinase deficiency. Biochim Biophys Acta 1360: 211–221, 1997
Magnani M, Stocchi V, Dachà M, Fornaini G: Regulatory properties of rabbit red blood cell hexokinase at conditions close to physiological. Biochim Biophys Acta 804: 145–153, 1984
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Magnani M, Bianchi M, Casabianca A, Stocchi V, Daniele A, Altruda F, Ferrone M, Silengo L: A recombinant human ‘mini’-hexokinase is catalytically active and regulated by hexose 6-phosphates. Biochem J 285: 193–199, 1992
Nishi S, Seino S, Bell GI: Human hexokinase: sequences of amino- and carboxyl-terminal halves are homologous. Biochem Biophys Res Commun 157: 937–943, 1988
Schwab DA, Wilson JE: Complete amino acid sequence of rat brain hexokinase, deduced from the cloned cDNA, and proposed structure of a mammalian hexokinase. Proc Natl Acad Sci USA 86: 2563–2567, 1989
Arora KK, Fanciulli M, Pedersen PL: Glucose phosphorylation in tumor cells. Cloning, sequencing, and overexpression in active form of a full-length cDNA encoding a mitochondrial bindable form of hexokinase. J Biol Chem 265: 6481–6488, 1990
Arora KK, Filburn CR, Pedersen PL: Structure/function relationships in hexokinase. Site-directed mutational analyses and characterization of overexpressed fragments implicate different functions for the N- and C-terminal halves of the enzyme. J Biol Chem 268: 18259–18266, 1993
Baijal M, Wilson JE: Residues putatively involved in binding of ATP and glucose 6-phosphate to a mammalian hexokinase: Site-directed mutation at analogous positions in the N- and C-terminal halves of the type I isozyme. Arch Biochem Biophys 321: 413–420, 1995
Sui D, Wilson JE: Functional interactions between the noncovalently associated N- and C-terminal halves of mammalian Type I hexokinase. Arch Biochem Biophys 401: 21–28, 2002
Hashimoto M, Wilson JE: Kinetic and regulatory properties of HK I(+), a modified form of the type I isozyme of mammalian hexokinase in which interactions between the N- and C-terminal halves have been disrupted. Arch Biochem Biophys 399: 109–115, 2002
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreoni, F., Serafini, G., Laguardia, M.E. et al. Bovine hexokinase type I: Full-length cDNA sequence and characterisation of the recombinant enzyme. Mol Cell Biochem 268, 9–18 (2005). https://doi.org/10.1007/s11010-005-1846-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-1846-5