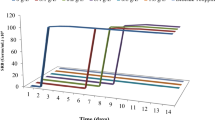
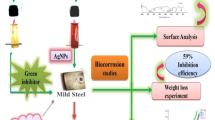
Guanidinium-containing oligomer based on aliphatic oligoepoxide is a newly synthesized substance with known bactericidal action, which is prospective as a microbial corrosion inhibitor. The oligomeric alkyl-substituted guanidine bromide is synthesized and its anticorrosive properties for steel in the presence of corrosive aggressive sulfate-reducing bacteria (SRB) are studied. The effectiveness of the new compound is compared with previously studied inhibitors DPH (quarter ammonium compound based on N-decylpyridinium chloride) (National Technical University, Kyiv, Ukraine) and Armohib CI-28 based on Diamine Ethoxylate (Akzonobel, Holland). The guanidine oligomer has biocidal properties. It significantly inhibits the growth of bacteria, and after the end of its exposure period only dozens of bacteria were found in the environment. The steel corrosion rate in the presence of SRB without inhibitors equals 0.15–0.35 mg/(cm2·h). The addition of DPH reduces the corrosion rate of steel to 0.032–0.047 mg/(cm2·h) (in 6.5–10.6 times). The addition of Armohib CI-28 reduces the corrosion rate to 0.027–0.039 mg/cm2·h (in 4.2–12.7 times). After the addition of the oligomer to the culture medium reduces with bacteria the corrosion rate decreases to 0.075–0.079 mg/(cm2·h) (in 2.5–2.7 times). According to the results of steel samples mass loss, the degree of the metal protection from microbial corrosion in the presence of guanidine oligomer is 60.15–63.17%. So, the guanidine-containing oligomer based on aliphatic oligoepoxide has biocidal and ant-corrosion properties and is promising for combating microbial induced corrosion.


Similar content being viewed by others
References
K. I. Andreyuk, I. P. Kozlova, Zh. P. Koptiayeva, A. I. Pidlyashenko-Novokhatyi, V. V. Zanina, and L. M. Purish, Microbial Corrosion of Underground Structures [in Ukrainian], Naukova Dumka, Kyiv (2005).
S. M. Bhola, F. M. Alabbas, and R. Bhola, “Neem extract as an inhibitor for biocorrosion influenced by sulfate reducing bacteria: A preliminary investigation,” Eng. Failure Analysis, 36, 92–103 (2014); https://doi.org/10.1016/j.engfailanal.2013.09.015.
L. M. Purish, D. R. Abdulina, and Iutynska, “Inhibitors of corrosion induced by sulfate-reducing bacteria,” Mikrobiol. Z., 83, Is. 6, 95–109 (2021); https://doi.org/10.15407/microbiolj83.06.095.
H. Hassan, A. Eldesoky, and W. Zordok, “Sulfa guanidine azo derivatives as environmentally – friendly corrosion inhibitors for nickel in HCl solution: theoretical and experimental study,” Int. J. of Sci. & Eng. Res., 5, Is. 5, 637–650 (2014).
K. F. Khaled, “New synthesized guanidine derivative as a green corrosion inhibitor for mild steel in acidic solutions,” Int. J. Electrochem. Sci., 3, Is. 4, 462–475 (2008).
K. F. Khaled, “Guanidine derivative as a new corrosion inhibitor for copper in 3% NaCl solution,” Mater. Chem. and Phys., 112, Is. 1, 104–111 (2008); https://doi.org/10.1016/j.matchemphys.2008.05.052.
M. Lebrini, F. Bentiss, N.-E. Chihib, C. Jama, J. P. Hornez, and M. Lagrenée, “Polyphosphate derivatives of guanidine and urea copolymer: Inhibiting corrosion effect of armco iron in acid solution and antibacterial activity,” Corr. Sci., 50, Is. 10, 2914–2918 (2008); https://doi.org/10.1016/j.corsci.2008.07.003.
A. Maitra, G. Singh, and B. Bijoy, “Study of corrosion inhibitor/stimulator characteristics of guanidine derivatives,” British Corr. J., 18, Is. 3, 152–155 (1983); https://doi.org/10.1179/000705983798273723.
V. B. Obraztsov, E. D. Rubl’ova, and N. V. Amirulloeva, “Influence of zinc ions on the inhibiting properties of polyhexamethylene guanidine derivatives,” Mater. Sci., 49, No. 3, 326–333 (2013); https://doi.org/10.1007/s11003-013-9618-y.
M. Ya. Vortman, Yu. B. Pysmenna, A. I. Chuenko, D. R. Abdulina, Zh. P. Kopteva, A. E. Kopteva, A. V. Rudenko, V. V. Tretyak, V. N. Lemeshko, and V. V. Shevchenko, “Fungicidal and bacterial activity of alkyl-substituted guanidine-containing oligomers,” Mikrobiolohichnyi Zhurnal, 82, Is. 6, 54–63 (2020); https://doi.org/10.15407/microbiolj82.06.054.
L. M. Purysh, I. A. Kozlova, and I. S. Pogrebova, “The effectiveness of the protective action of steel corrosion inhibitors in the presence of sulfate-reducing bacteria,” Praktika Protivokor. Zashchity, 67, No. 1, 18–24 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 58, No. 5, pp. 17–22, September–October, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdulina, D.R., Vortman, M.Y., Iutynska, G.A. et al. Guanidine-Containing Alkyl-Substituted Oligomer as a Metal Corrosion Inhibitor. Mater Sci 58, 579–584 (2023). https://doi.org/10.1007/s11003-023-00701-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-023-00701-6