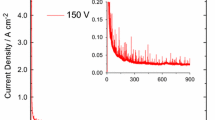
Earlier, it was shown that titanium in phosphoric-acid solutions has a restricted corrosion resistance. The aim of our investigation is to study the electrochemical behavior of pressed porous titanium structures in phosphoric acid in the presence of ions of heavy metals. By using the volumetric and potentiometric methods and the method of cyclic voltammetry, we investigate the electrochemical processes on the surface of porous titanium electrodes in solutions of phosphoric acid. The addition of surface-active organic compounds with sulfur- and nitrogen-containing groups only insignificantly delays the onset of the corrosion process, which reveals a low efficiency of the organic corrosion inhibitors for titanium. It is demonstrated that Cu2+ -ions can be effective inhibitors for porous titanium structures. In addition to the blocking effect copper forms an independent phase and may play the role of cathodic additive. The overvoltage of hydrogen release for copper is much lower than for titanium, which facilitates the cathodic process and results in a shift of the potential of corrosion couple into the zone of passivation of titanium.




Similar content being viewed by others
References
O. V. Kosohin, A. I. Kushmyruk, Yu. S. Miroshnychenko, and O. V. Linyucheva, “Electrochemical properties of titanium-based catalytically active electrodes in perchloric acid,” Fiz.-Khim. Mekh. Mater., 48, No. 2, 18–24 (2012); English translation : Mater. Sci., 48, No. 2, 139–146 (2012).
V. P. Chviruk, E. M. Zaverach, and O. V. Linyucheva, “Behavior of porous titanium and catalytically active electrodes based on it in acidic chloride solutions,” Russ. J. Electrochem., 37, No. 5, 512–517 (2001).
A. I. Kushmyruk, O. V. Kosohin, O. V. Linyucheva, V. A. Reveko, and Yu. S. Miroshnychenko, “Electrochemical behavior of porous titanium electrodes in phosphoric acid,” Fiz.-Khim. Mekh. Mater., 51, No. 3, 121–126 (2015); English translation : Mater. Sci., 51, No. 3, 429–435 (2015).
U. Kuhn, H. Kiesele, and S. Haupt, Electrochemical Measuring Cell for Detecting Hydrogen Cyanide or Sulfur Dioxide, Patent US 5041204, Int.Cl5 G 01 N 27/49, G 01 N 27/40, Dragerwerk Aktiegesellschoft, No. 07/543,436, 26.06.1990, 20.08.1991.
S. Sommer, H. Kiesele, and F. Mett, Electrochemical Sensor Having a Mediator Compound with a Solid, Patent US 8268161, Int.Cl5 G 01 N 27/49, G 01 N 27/40, Drägerwerk AG & Co. KGaA, No. 11/677,782, 22.02.2007, 18.09.2012.
N. V. Shel’, L. E. Tsygankova, and V. I. Vigdorovich, “Influence of Cu2+ and Ce4+ ions on the delay of corrosion and the anodic ionization of titanium in 4 М solutions of sulfuric acid with HF,” Zashch. Metal., 33, No. 2, 164–167 (1997).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 52, No. 5, pp. 66–70, September–October, 2016.
Rights and permissions
About this article
Cite this article
Kushmyruk, A.I., Linyucheva, O.V., Kosohin, O.V. et al. Electrochemical Behavior of Porous Titanium Structures in Phosphoric Acid in the Presence of Ions of Copper (ІІ). Mater Sci 52, 675–679 (2017). https://doi.org/10.1007/s11003-017-0008-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-017-0008-8