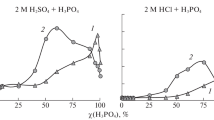
We study the conditions of appearance of synergistic effects in inhibiting mixtures aimed at the protection of steel against corrosion in neutral aqueous-salt and acid media. It is shown that the high anticorrosion efficiency in neutral media is attained by the application of inhibitors with different mechanisms of action guaranteeing the efficient passivation in a broad range of potentials. We propose an approach to the optimization of complexing synergistic compositions for the protection of steel in acid media, which enables one to increase their efficiency as a result of the improvement of the microstructure of the phase layer. It is shown that the method of isomolar series opens wide possibilities for the investigation of the nature and conditions of the formation of synergistic effects.





Similar content being viewed by others
References
Yu. V. Fedorov, “On the action of inhibiting mixtures,” in: Proc. of the Third Internat. Congress on the Corrosion of Metals [in Russian], Vol. 2, Mir, Moscow (1968), pp. 150–162.
Z. A. Iofa, “Effects of synergism and antagonism in the adsorption and action of surface-active substances on the electrochemical reactions and the corrosion of iron,” Zashch. Met., 8, No. 2, 139–145 (1972).
L. I. Antropov, I. S. Pogrebova, and G. I. Dremova, “On the combined influence of haloid ions and quaternary salts with pyridine bases on the acid corrosion of metals,” Élektrokhimiya, 8, 108–112 (1972).
G. Wiecsorec and Z. Szklarska-Smialowska, “Synergetic action of n-dodecilamine with n-capric acid on the corrosion of steel in sulfuric acid,” Corr. Sci., 12, 877–889 (1972).
A. K. Mindyuk, E. N. Svist, and A. N. Gopanenko, “Efficiency of the combined protection of steel in sulfuric acid by inhibitors and surface-active anions,” Korr. Zashch. Neftegaz. Prom., No. 2, 6–9 (1975).
I. D. Rozenfel’d, Corrosion Inhibitors [in Russian], Khimiya, Moscow (1977).
L. I. Antropov, E. M. Makushin, and V. F. Panasenko, Inhibitors of the Corrosion of Metals [in Russian], Tekhnika, Kiev (1981).
V. M. Ledovskikh, “Synergistic inhibition of the acid corrosion of steel,” Zashch. Met., 20, No. 1, 54–61 (1984).
I. D. Rozenfel’d, L. V. Frolova, and N. N. Tavadze, “Synergistic effect in the protection of steel against corrosion in neutral electrolytes by inorganic inhibitors,” Zashch. Met., 16, No. 2, 133–136 (1980).
V. M. Ledovskikh, “Synergistic inhibition of the corrosion of steel in neutral media by the compositions of nitrogen organic bases with sodium nitrite,” Zashch. Met., 19, No. 1, 84–91 (1983).
V. M. Ledovskikh and N. F. Kuleshova, “Inhibition of metal corrosion in neutral media,” Vestn. Kiev. Politekh. Inst., Ser. Khim. Mashinostr. Tekhnol., Issue 19, 59–61 (1982).
V. M. Ledovs’kykh, S. V. Levchenko, and S. M. Tulainov, “Synergistic extrema of the mixtures of corrosion inhibitors for metals in aqueous salt solutions,” Fiz.-Khim. Mekh. Mater., 49, No. 6, 107–111 (2013): English translation: Mater. Sci., 49, No. 6, 827–832 (2013).
V. M. Ledovskykh, Yu. P. Vyshnevska, S. K. Poznyak, I. V. Brazhnyk, and S. V. Levchenko, “Inhibition mechanism, analytic prediction, and purposeful design of highly efficient mixed-type corrosion inhibitors,” Fiz.-Khim. Mekh. Mater., Vol. 2, Special Issue No. 10, 416–420 (2014).
M. I. Donchenko, S. V. Frolenkova, O. H. Sribna, and N. A. Bilousova, “Passivating treatment of iron and low-carbon steel for the temporal protection against atmospheric corrosion,” Fiz.-Khim. Mekh. Mater., Special Issue No. 6, 124–129 (2007).
Yu. P. Vishnevska and I. V. Brazhnyk, “Mechanism and kinetics of phase protective layers formation under various pH and temperature conditions,” in: 18th Internat. Conf. on the Corrosion of Underground Structures (Košice, Slovakia, May 23–24, 2013) (2013), pp. 58–62.
M. Valiev, E. J. Bylaska, N. Govind, K. Kowalski, T. P. Straatsma, H. J. J. van Dam, D. Wang, J. Nieplocha, E. Apra, T. L. Windus, and W. A. de Jong, “NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations,” Comput. Phys. Comm., 181, 1477 (2010).
N. P. Zhuk, A Course of the Theory of Corrosion and Protection of Metals [in Russian], Metallurgiya, Moscow (1976).
D. A. Tkalenko, V. O. Lavrenko, and Yu. P. Vyshnevs’ka, “Polarization resistance in the formation of protective phase layers with participation of organic ligands,” Dopov. Nats. Akad. Nauk Ukr., No. 12, 120–126 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 52, No. 5, pp. 30–37, September–October, 2016.
Rights and permissions
About this article
Cite this article
Ledovs’kykh, V.М., Vyshnevs’ka, Y.P., Brazhnyk, І.V. et al. Development and Optimization of Synergistic Compositions for the Corrosion Protection of Steel in Neutral and Acid Media. Mater Sci 52, 634–642 (2017). https://doi.org/10.1007/s11003-017-0002-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-017-0002-1