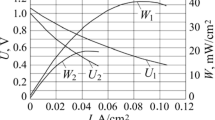
We study the influence of reducing and oxidizing media on the mutual diffusion of chemical elements between the (Ce,Gd)O2–δ barrier (protective) cathodic layer and the YSZ electrolyte of a solid-oxide fuel cell in the course of annealing at 1400°С. The results of the analysis of distributions of chemical elements in annealed specimens demonstrate that, in the case of annealing in the reducing medium, the diffusion of Ce and Gd into the electrolyte is more intense than the diffusion of Zr from the electrolyte into the protective layer. As compared with the oxidizing medium (air), the mutual diffusion of Ce, Gd, and Zr in the reducing (hydrogen-containing) medium is much more intense and the depth of their penetration is 1.5–3 times larger.





Similar content being viewed by others
References
A. Mai, V. Haanappel, S. Uhlenbruck, F. Tietz, and D. Stоеver, “Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells. Part I. Variation of composition,” Solid State Ionics, 176, 1341–1350 (2005).
A. Mai, V. Haanappel, F. Tietz, and D. Stoever, “Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells. Part II. Influence of the CGO interlayer,” Solid State Ionics, 177, 2103–2107 (2006).
F. Tietz, V. Haanappel, A. Mai, J. Mertens, and D. Stoever, “Performance of LSCF cathodes in cell tests,” J. Power Sources, 156, 20–22 (2006).
X.-D. Zhou, B. Scarfino, and H. U. Anderson, “Electrical conductivity and stability of Gd-doped ceria/Y-doped zirconia ceramics and thin films,” Solid State Ionics, 175. 19–22 (2004).
H. Mitsuyasu, Y. Nonaka, and K. Eguchi, “Analysis of solid state reaction at the interface of yttria-doped ceria/yttria-stabilized zirconia,” Solid State Ionics, 113–115, 279–284 (1998).
H. Yokokawa, N. Sakai, T. Horita, K. Yamai, M. E. Brito, and H. Kishimoto, “Thermodynamic and kinetic considerations on degradations in solid oxide fuel cell cathodes,” J. Alloys Compound., 452, No. 1, 41–47 (2008).
T. Kawada and J. Mizusaki, “Current electrolytes and catalyst,” in: W. Vielstich, A. Lamm, and H. A. Gasteiger (editors), Handbook of Fuel Cells: Fundamentals, Technology, Applications, Wiley, Chichester (2003), pp. 987–1001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 51, No. 4, pp. 107–113, July–August, 2015.
Rights and permissions
About this article
Cite this article
Ushkalov, L.M., Brodnikovs’kyi, E.М., Lysunenko, N.О. et al. Diffusion Processes Between the Barrier Cathodic Layer and the Electrolyte of a Solid-Oxide Fuel Cell. Mater Sci 51, 555–562 (2016). https://doi.org/10.1007/s11003-016-9875-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-016-9875-7