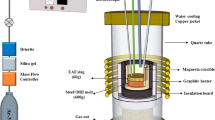
By the method of high-energy milling in a ball mill, we obtain new alloys of Mg–M−Ni (M = Al, Mn, Ti) ternary systems. The properties of hydrogen sorption of the Mg3AlNi2 compound (Ti2Ni-type structure) are investigated and compared with the properties of Mg3MNi2 (M = Mn, Ti) isostructural compounds. The sorption–desorption of hydrogen by Mg88M4Ni8 (M = Al, Mn, Ti) alloys is studied. The catalytic influence of Mg3MNi2 ternary phases on the hydrogenation of magnesium is established.










Similar content being viewed by others
References
B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher, “Metal hydride materials for solid hydrogen storage: A review,” Int. J. Hydrogen Energy, 32, 1121–1140 (2007).
R. A. Varin, T. Czujko, and Z. S. Wronski, Nanomaterials for Solid State Hydrogen Storage, Springer, New York (2009).
A. Zaluska, L. Zaluski, and J. O. Ström-Olsen, “Nanocrystalline magnesium for hydrogen storage,” J. Alloys Comp., 288, 217–225 (1999).
І. Yu. Zavalii, R. V. Denys, and V. V. Berezovets,’ “Mechanochemical methods for the synthesis of new magnesium-based composite materials for hydrogen accumulation,” Fiz.-Khim. Mekh. Mater., 45, No. 2, 93–101 (2009); English translation: Mater. Sci., 45, No. 2, 248–257 (2009).
R. V. Denys, V. V. Berezovets,’ and І. Yu. Zavalii, “Hydrogen-sorbing materials based on magnesium,” in: V. D. Pokhodenko, V. V. Skorokhod, and Yu. M. Solonin (editors), Fundamental Problems of Hydrogen Power Engineering [in Ukrainian], KIM, Kyiv (2010), pp. 245–265.
G. Liang, J. Huot, S. Boily, et al., “Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–TM (TM = Ti, V, Mn, Fe and Ni) systems,” J. Alloys Comp., 292, 247–252 (1999).
S. Bouaricha, J. P. Dodelet, D. Guay, et. al., “Hydriding behavior of Mg–Al and leached Mg–Al compounds prepared by high energy ball-milling,” J. Alloys Comp., 297, 282–293 (2002).
A. Andreasen, M. B. Sorensen, R. Burkarl, et al., “Interaction of hydrogen with an Mg–Al alloy,” J. Alloys Comp., 404–406, 323–326 (2005).
H. Blomqvist, Magnesium Ions Stabilizing Solid-State Transition Metal Hydrides, Doctoral Dissertation, Institutionen for Fysikalisk kemi, Organisk kemi och Strukturkemi Stockholms Universitet, Stockholm (2003).
J. J. Didisheim, P. Zolliker, K. Yvon, et al., “Dimagnesium iron (II) hydride, Mg2FeH6, containing octahedral FeH6 4- anions,” Inorg. Chem., 23, 1953–1957 (1984).
J. Huot, H. Hayakawa, and E. Akiba, “Preparation of the hydrides Mg2FeH6 and Mg2CoH5 by mechanical alloying followed by sintering,” J. Alloys Comp., 248, 164–167 (1997).
M. Dornheim, S. Doppiu, G. Barkhordarian, et al., “Hydrogen storage in magnesium-based hydrides and hydride composites,” Scr. Mater., 56, 5841–5846 (2007).
Y. Zhang, H. Yang, H. Yuan, et al., “Dehydriding properties of ternary Mg2Ni1–x Zr x hydrides synthesized by ball milling and annealing,” J. Alloys Comp., 269, 278–283 (1998).
Y. Takahashi, H. Yukawa, and M. Morinaga, “Alloying effects on the electronic structure of Mg2Ni intermetallic hydride,” J. Alloys Comp., 242, 98–107 (1996).
G. Lu, L. Chen, L. Wang, et al., “Study on the phase composition of Mg2–x M x Ni (M = Al, Ti) alloy,” J. Alloys Comp., 321, L1–L4 (2001).
F. C. Gennari, G. Urretavizcaya, J. J. Andrade Gamboa, et al., “New Mg-based alloy obtained by mechanical alloying in the Mg–Ni–Ge system,” J. Alloys Comp., 354, 187–192 (2003).
R. V. Denys, I. Yu. Zavaliy, V. Paul-Boncour, et al., “New Mg–Mn–Ni alloys as efficient hydrogen storage materials,” Intermetallics, 18, 1579–1585 (2010).
R. V. Denys, I. Yu. Zavaliy, V. V. Berezovets, et al., “Phase equilibria in the Mg–Ti–Ni system at 500°C and hydrogenation properties of selected alloys,” Intermetallics, 32, 167–175 (2013).
R. V. Denys, A. R. Riabov, V. V. Berezovets, et al., “Crystal structure of the novel Mg3MnNi2D3−x interstitial deuteride,” Intermetallics, 19, 1563–1566 (2011).
G. Liang, S. Boily, J. Huot, et al., “Mechanical alloying and hydrogen absorption properties of the Mg–Ni system,” J. Alloys Comp., 267, 302–306 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 49, No. 2, pp. 26–34, March–April, 2013.
Rights and permissions
About this article
Cite this article
Berezovets’, V.V., Denys, R.V., Zavalii, I.Y. et al. Characteristic Features of the Sorption–Desorption of Hydrogen by Mg–M–Ni (M = Al, Mn, Ti) Ternary Alloys. Mater Sci 49, 159–169 (2013). https://doi.org/10.1007/s11003-013-9595-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-013-9595-1