Abstract
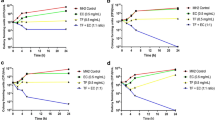
Antimicrobial peptides (AMPs) are promising candidates for the development of new drugs. However, thorough studies on the toxicity of these molecules are scarce, which is a gap, as host toxicity is one of the main reasons for nonapproval of the drug by regulatory agencies. This work aimed to evaluate the toxicity of three AMPs isolated from Capsicum annuum leaves, named CaCPin-II, CaCDef-like and CaCLTP2. The AMP toxicological profile was evaluated by in vitro cytotoxicity against mammalian cells and systemic in vivo toxicity using Galleria mellonella larvae as study model. AMP cytotoxicity was evaluated in a broad panel of human cell lines, namely, vascular endothelium, cervical adenocarcinoma, prostatic epithelium, mammary epithelium and fibroblasts, and in murine macrophages. Cell viability was evaluated through metabolic activity, a gold standard method for assessing viability due to the speed, robustness and reliability of the results. To elucidate the toxicity mechanism of the peptides, their ability to bind to the cell surface and to permeabilize membranes was evaluated by measuring the zeta potential and the absorption of the SYTOX® Green fluorescent probe, respectively. The AMPs did not decrease cell viability or permeabilize the membranes of the cell lines at the tested concentrations. Only CaCLTP2 had the ability to interact with the cell surface, but it was not able to permeabilize them. The in vivo systemic toxicity was evaluated by the survival rate of the G. mellonella larvae inoculated with peptides. CaCPin-II showed in vivo toxicity, as the larval survival rate after the test was 60% lower than that of the controls. The results suggest that these peptides have potential as antimicrobial agents because they have low or no toxicity to mammalian cells and can serve as a framework for drug development.





Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Aerts AM, François IEJA, Meert EMK et al (2007) The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J Mol Microbiol Biotechnol 13:243–247. https://doi.org/10.1159/000104753
Allegra E, Titball RW, Carter J, Champion OL (2018) Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 198:469–472. https://doi.org/10.1016/j.chemosphere.2018.01.175
Almeida CV, de Oliveira CFR, dos Santos EL et al (2021) Differential interactions of the antimicrobial peptide, RQ18, with phospholipids and cholesterol modulate its selectivity for microorganism membranes. Biochim Biophys Acta - Gen Subj 1865https://doi.org/10.1016/j.bbagen.2021.129937.
Buck AK, Elmore DE, Darling LEO (2019) Using fluorescence microscopy to shed light on the mechanisms of antimicrobial peptides. Future Med Chem 11:2445–2458. https://doi.org/10.4155/fmc-2019-0095
Camini FC, da Silva Caetano CC, Almeida LT, de Brito Magalhães CL (2017) Implications of oxidative stress on viral pathogenesis. Arch Virol 162:907–917. https://doi.org/10.1007/s00705-016-3187-y
Cardoso MH, Orozco RQ, Rezende SB et al (2020) Computer-aided design of antimicrobial peptides: are we Generating Effective Drug candidates? Front Microbiol 10:1–15. https://doi.org/10.3389/fmicb.2019.03097
Carvalho A, de O, Gomes VM (2012) Plant defensins and defensin-like peptides - Biological activities and Biotechnological Applications. Curr Pharm Des 17:4270–4293. https://doi.org/10.2174/138161211798999447
Chen CH, Lu TK (2020) Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9. https://doi.org/10.3390/antibiotics9010024
Cherene MB, Taveira GB, Almeida-Silva F et al (2023a) Structural and biochemical characterization of three antimicrobial peptides from Capsicum annuum L. var. annuum leaves for anti – Candida Use. https://doi.org/10.1007/s12602-023-10112-3. Probiotics Antimicrob Proteins
Cherene MB, Ferreira SR, dos Santos L A, et al (2023b) Insecticidal activity of Capsicum annuum L. leaf proteins on cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae) development. J Asia Pac Entomol 26:1–11. https://doi.org/10.1016/j.aspen.2023.102158
Choi H, Rangarajan N, Weisshaar JC (2016) Lights, Camera, Action! Antimicrobial peptide mechanisms imaged in space and time. Trends Microbiol 24:111–122. https://doi.org/10.1016/j.tim.2015.11.004
Cutuli MA, Petronio Petronio G, Vergalito F et al (2019) Galleria mellonella as a consolidated in vivo model hosts: new developments in antibacterial strategies and novel drug testing. Virulence 10:527–541. https://doi.org/10.1080/21505594.2019.1621649
da Cunha NB, Cobacho NB, Viana JFC et al (2017) The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov Today 22:234–248. https://doi.org/10.1016/j.drudis.2016.10.017
Edqvist J, Blomqvist K, Nieuwland J, Salminen TA (2018) Plant lipid transfer proteins: are we finally closing in on the roles of these enigmatic proteins? J Lipid Res 59:1374–1382. https://doi.org/10.1194/jlr.R083139
Farag MR, Alagawany M (2018) Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem Biol Interact 279:78–83. https://doi.org/10.1016/j.cbi.2017.11.007
Finkina EI, Melnikova DN, Bogdanov IV, Ovchinnikova TV (2016) Lipid transfer proteins as components of the plant innate immune system: structure, functions, and applications. Acta Naturae 8:47–61. https://doi.org/10.32607/20758251-2016-8-2-47-61
Freire JM, Gaspar D, Veiga AS, Castanho MARB (2015) Shifting gear in antimicrobial and anticancer peptides biophysical studies: from vesicles to cells. J Pept Sci 21:178–185. https://doi.org/10.1002/psc.2741
Gebara RdaS, Taveira GB, de Azevedo dos Santos L et al (2020) Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotics Antimicrob Proteins 12:1253–1265. https://doi.org/10.1007/s12602-020-09647-6
Greco I, Molchanova N, Holmedal E et al (2020) Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-69995-9
Gupta S, Kapoor P, Chaudhary K et al (2015) Peptide Toxicity Prediction. In: Computational Peptidology. pp 143–57
Harris F, Dennison SR, Singh J, Phoenix DA (2011) On the selectivity and efficacy of defense peptides with respect to Cancer cells. Med Res Rev 33:190–234. https://doi.org/10.1002/med.20252
Hein MJA, Kvansakul M, Lay FT et al (2022) Defensin-lipid interactions in membrane targeting: mechanisms of action and opportunities for the development of antimicrobial and anticancer therapeutics. Biochem Soc Trans 50:423–437. https://doi.org/10.1042/BST20200884
Helmerhorst EJ, Reijnders IM, Van Hof ’T W, et al (1999) A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Lett 449:105–110. https://doi.org/10.1016/S0014-5793(99)00411-1
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:1–21. https://doi.org/10.3389/fmicb.2020.582779
Huang HW (2006) Molecular mechanism of antimicrobial peptides: the origin of cooperativity. 1758:1292–1302. https://doi.org/10.1016/j.bbamem.2006.02.001
Khabbaz H, Karimi-Jafari MH, Saboury AA, BabaAli B (2021) Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinformatics 22:1–11. https://doi.org/10.1186/s12859-021-04468-y
Khan F, Niaz K, Abdollahi M (2018) Toxicity of biologically active peptides and future safety aspects: an update. Curr Drug Discov Technol 15:236–242. https://doi.org/10.2174/1570163815666180219112806
Koo HB, Seo J (2019) Antimicrobial peptides under clinical investigation. Pept Sci 111. https://doi.org/10.1002/pep2.24122
Kovaleva V, Bukhteeva I, Kit OY, Nesmelova IV (2020) Plant defensins from a structural perspective. Int J Mol Sci 21:1–23. https://doi.org/10.3390/ijms21155307
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343. https://doi.org/10.1016/j.freeradbiomed.2009.05.004
Kulkarni MM, Mcmaster WR, Kamysz W, Mcgwire BS (2009) Antimicrobial peptide-induced apoptotic death of Leishmania results from Calcium-dependent, caspase-independent mitochondrial toxicity *. J Biol Chem 284:15496–15504. https://doi.org/10.1074/jbc.M809079200
Lei J, Sun LC, Huang S et al (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11:3919–3931
Lewies A, Du Plessis LH, Wentzel JF (2019) Antimicrobial peptides: the Achilles’ heel of Antibiotic Resistance? Probiotics Antimicrob Proteins 11:370–381. https://doi.org/10.1007/s12602-018-9465-0
Li S, Wang Y, Xue Z et al (2021) The structure-mechanism relationship and mode of actions of antimicrobial peptides: a review. Trends Food Sci Technol 109:103–115. https://doi.org/10.1016/j.tifs.2021.01.005
Maximiano MR, Franco OL (2021) Biotechnological applications of versatile plant lipid transfer proteins (LTPs). https://doi.org/10.1016/j.peptides.2021.170531. Peptides 140:
Mello EO, Ribeiro SFF, Carvalho AO et al (2011) Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr Microbiol 62:1209–1217. https://doi.org/10.1007/s00284-010-9847-3
Melnikova DN, Finkina EI, Bogdanov IV et al (2023) Features and possible applications of plant lipid-binding and transfer proteins. Membr (Basel) 13:1–17
Mishra M, Tamhane VA, Khandelwal N et al (2010) Interaction of recombinant CanPIs with Helicoverpa armigera gut proteases reveals their processing patterns, stability and efficiency. Proteomics 10:2845–2857. https://doi.org/10.1002/pmic.200900853
Mohs RC, Greig NH (2017) Drug discovery and development: role of basic biological research. Alzheimer’s Dement Transl Res Clin Interv 3:651–657. https://doi.org/10.1016/j.trci.2017.10.005
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19:311–332. https://doi.org/10.1038/s41573-019-0058-8
Moretta A, Scieuzo C, Petrone AM et al (2021) Antimicrobial peptides: a New Hope in Biomedical and Pharmaceutical Fields. Front Cell Infect Microbiol 11:1–26. https://doi.org/10.3389/fcimb.2021.668632
Mylonakis E, Moreno R, Khoury JB, El et al (2005) Galleria mellonella as a Model System to study Cryptococcus neoformans Pathogenesis. Infect Immun 73:3842–3850. https://doi.org/10.1128/IAI.73.7.3842
Ojeda PG, Cardoso MH, Franco OL (2019) Pharmaceutical applications of cyclotides. Drug Discov Today 24:2152–2161. https://doi.org/10.1016/j.drudis.2019.09.010
Oliveira FD, Cavaco M, Figueira TN et al (2022) The antimetastatic breast cancer activity of the viral protein-derived peptide vCPP2319 as revealed by cellular biomechanics. FEBS J 289:1603–1624. https://doi.org/10.1111/febs.16247
Piatek M, Sheehan G, Kavanagh K (2021) Galleria mellonella: the versatile host for drug discovery, in vivo toxicity testing and characterising host-pathogen interactions. Antibiotics 10. https://doi.org/10.3390/antibiotics10121545
Pognan F, Beilmann M, Boonen HCM et al (2023) The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov 22:317–335. https://doi.org/10.1038/s41573-022-00633-x
Robles-Loaiza AA, Pinos-Tamayo EA, Mendes B et al (2022) Traditional and computational screening of non-toxic peptides and approaches to improving selectivity. Pharmaceuticals 15. https://doi.org/10.3390/ph15030323
Rodríguez-Rojas A, Makarova O, Rolff J (2014) Antimicrobials, stress and Mutagenesis. PLoS Pathog 10. https://doi.org/10.1371/journal.ppat.1004445
Rudzińska M, Daglioglu C, Savvateeva LV et al (2021) Current status and perspectives of protease inhibitors and their combination with nanosized drug delivery systems for targeted cancer therapy. Drug Des Devel Ther 15:9–20. https://doi.org/10.2147/DDDT.S285852
Ruiz J, Calderon J, Rondón-Villarreal P, Torres R (2014) Analysis of structure and hemolytic activity relationships of antimicrobial peptides (AMPs). Adv Intell Syst Comput 232:253–258. https://doi.org/10.1007/978-3-319-01568-2_36
Salminen TA, Blomqvist K, Edqvist J (2016) Lipid transfer proteins: classification, nomenclature, structure, and function. Planta 244:971–997. https://doi.org/10.1007/s00425-016-2585-4
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625:190–194. https://doi.org/10.1016/j.ejphar.2009.08.043
Serrano I, Verdial C, Tavares L, Oliveira M (2023) The virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 12:505. https://doi.org/10.3390/antibiotics12030505
Seyfi R, Kahaki FA, Ebrahimi T et al (2020) Antimicrobial peptides (AMPs): roles, functions and mechanism of action. Int J Pept Res Ther 26:1451–1463. https://doi.org/10.1007/s10989-019-09946-9
Sivertsen A, Isaksson J, Leiros HKS et al (2014) Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct Biol 14. https://doi.org/10.1186/1472-6807-14-4
Skalska J, Andrade VM, Cena GL et al (2020) Synthesis, structure, and activity of the Antifungal Plant Defensin PvD1. J Med Chem 63:9391–9402. https://doi.org/10.1021/acs.jmedchem.0c00543
Soares JR, José Tenório de Melo E, da Cunha M et al (2017) Interaction between the plant ApDef1 defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle- and caspase-dependent process occurring via uncontrolled oxidative stress. Biochim Biophys Acta - Gen Subj 1861:3429–3443. https://doi.org/10.1016/j.bbagen.2016.09.005
Sok M, Šentjurc M, Schara M (1999) Membrane fluidity characteristics of human lung cancer. Cancer Lett 139:215–220. https://doi.org/10.1016/S0304-3835(99)00044-0
Svenson J, Brandsdal BO, Stensen W, Svendsen JS (2007) Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J Med Chem 50:3334–3339. https://doi.org/10.1021/jm0703542
Tamimi NAM, Ellis P (2009) Drug development: from concept to marketing! Nephron - Clin Pract 113:125–131. https://doi.org/10.1159/000232592
Van Vliet E (2011) Current standing and future prospects for the technologies proposed to transform toxicity testing in the 21st century. Altex 28:17–44. https://doi.org/10.14573/altex.2011.1.017
Vieira-da-Silva B, Castanho MARB (2023) Resazurin reduction-based assays revisited: guidelines for Accurate Reporting of relative differences on metabolic status. Molecules 28. https://doi.org/10.3390/molecules28052283
Wei D, Zhang X (2022) Biosynthesis, bioactivity, biotoxicity and applications of antimicrobial peptides for human health. Biosaf Heal 4:118–134. https://doi.org/10.1016/j.bsheal.2022.02.003
Yadav NK, Saikhedkar NS, Giri AP (2021) PINIR: a comprehensive information resource for Pin-II type protease inhibitors. BMC Plant Biol 21
Yeaman MR, Büttner S, Thevissen K (2018) Regulated cell death as a therapeutic target for novel antifungal peptides and biologics. Oxid Med Cell Longev 2018.https://doi.org/10.1155/2018/5473817.
Zasloff M (2019) Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol 1117:3–6. https://doi.org/10.1007/978-981-13-3588-4_1
Zhang QY, Yan Z, Bin, Meng YM et al (2021) Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res 8:1–25. https://doi.org/10.1186/s40779-021-00343-2
Acknowledgements
This work was performed at the Universidade de Lisboa and Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF).
Funding
We acknowledge the financial support of the Brazilian agencies CNPq (307590/2021-6), FAPERJ (E-26/200567/2023; E-26/210353/2022; E-26/200.127/2023) and European Union H2020-01 (828774). This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), finance code 001.
Author information
Authors and Affiliations
Contributions
The study was conceived by MBC, MARBC and VMG. Experimental procedures were carried out by MBC, GBT, MCC, TZAG, LAS, EOM. Data analyses were performed by MBC, GBT, MCC, VLSN, AOC, MARBC. The paper was written by MBC, MCC, GBT, VMG, MARBC. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cherene, M.B., Cavaco, M.C., Neves, V.L.S. et al. Non-toxicity of Plant Candicidal Peptides for Mammalian Cell Lines and Galleria mellonella Model to Improving Selectivity for Clinical Use. Int J Pept Res Ther 30, 28 (2024). https://doi.org/10.1007/s10989-024-10607-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-024-10607-9