Abstract
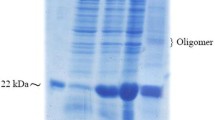
Human truncated parathyroid hormone [hPTH (1–34)], a peptide hormone which accelerated the research interest towards the theraputical applications. This study outlines the effective expression of [hPTH (1–34)] in Escherichia coli and the recuperation of highly soluble truncated PTH from the fusion protein by proteolytic digestion. Successful expression of glutathione S-transferase (GST) fusion protein was achieved by incorporating truncated PTH with an enzymatic cleavage site into the “N” terminal GST of pGEX-4T-3 expression vector. Under the optimized condition, we achieved more than 120 mg of pure hPTH (1–34) per liter of bacterial culture, with an overall yield of 39%. Purification process was carried out through immobilized metal ion chromatography and membrane filter to produce maximum purity. Physical characterization using western blot analysis showed that the extracted truncated PTH is intact and reacts with anti-PTH antibodies. In vitro analysis of PTH stimulated adenylyl cyclase activation in UMR 106 cells confirmed biological activity for purified protein. We believe this vector and production methodology represents a generally applicable tool for the generation of recombinant peptides.







Similar content being viewed by others
References
Al-Badran AE, Abdul-Jabbar RA (2017) Extraction and purification of recombinant intact human parathyroid horman (hPTH) from bacterial cell. JBEI 3:1–10. https://doi.org/10.5430/jbei.v3n2p1
Audu CO, Cochran JC, Pellegrini M, Mierke DF (2013) Recombinant production of TEV cleaved human parathyroid hormone. J Pept Sci 19:504–510. https://doi.org/10.1002/psc.2528
Baron R, Hesse E (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 97(2):311–325. https://doi.org/10.1210/jc.2011-2332
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chilakapati R, Mannully CT, Pulicherla KK (2018) Prospects of parathyroid hormone in therapeutic intervention. Int J Pept Res Ther. https://doi.org/10.1007/s10989-018-9744-3
Cupp ME, Nayak SK, Adem AS, Thomsen WJ (2013) Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J Pharmacol Exp Ther 345(3):404–418. https://doi.org/10.1124/jpet.112.199752
Dobnig H, Turner RT (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612. https://doi.org/10.1210/endo.138.11.5505
Dong S, Shang S, Li J, Tan Z, Dean T, Maeda A, Gardella TJ, Danishefsky SJ (2012) Engineering of therapeutic polypeptides through chemical synthesis: early lessons from human parathyroid hormone and analogues. J Am Chem Soc 134:15122–15129. https://doi.org/10.1021/ja306637u
Ertl DA, Stary S, Streubel B, Raimann A, Haeusler G (2012) A novel homozygous mutation in the parathyroid hormone gene (PTH) in a girl with isolated hypoparathyroidism. Bone 51(3):629–632. https://doi.org/10.1016/j.bone.2012.06.009
Gangireddy SR, Madhavi RD, Ravikanth KR, Reddy PK, Konda VR, Rao KRS, Gunwar S (2010) High yield expression of human recombinant PTH (1-34). Curr Trends Biotechnol Pharm 4(1):568–577
Gera M, Kim N, Ghosh M, Sharma N, Huynh DL, Chandimali N, Koh H, Zhang JJ, Kang TY, Park YH, Kwon T, Jeong DK (2019) Synthesis and evaluation of the antiproliferative efficacy of BRM270 phytocomposite nanoparticles against human hepatoma cancer cell lines. Mater Sci Eng C 97:166–176. https://doi.org/10.1016/j.msec.2018.11.055
Ghosh M, Pulicherla KK, Rekha V, Rao GV, Rao KRSS (2012) A review on successive generations of streptokinase based thrombolytic agents. Int J Pharm Pharm Sci 4(3):38–42
Ghosh, M, Sharma N, Gera M, Kim N, Sodhi SS, Pulicherla KK, Huynh D, Kim DC, Zhang J, Kwon T, Do KT, Lee HK, Song KD, Jeong DK (2018a) The first comprehensive description of the expression profile of genes involved in differential body growth and the immune system of the Jeju Native Pig and miniature pig. Amino Acids. https://doi.org/10.1007/s00726-018-2685-5
Ghosh M, Sharma N, Singh AK, Gera M, Pulicherla KK, Jeong DK (2018b) Transformation of animal genomics by next-generation sequencing technologies: a decade of challenges and their impact on genetic architecture. Crit Rev Biotechnol 38(8):1157–1175. https://doi.org/10.1080/07388551.2018.1451819
Habener JF, Rosenblatt M, Potts JT Jr (1984) Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev 64:985–1053. https://doi.org/10.1152/physrev.1984.64.3.985
Hamedifar H, Salamat F, Saffarion M, Ghiasi M, Hosseini A, Lahiji H, Nouri Z, Arfae H, Mahboudi F (2013) A novel approach for high level expression of soluble recombinant human parathyroid hormone (rhPTH 1-34) in Escherichia coli. Avicenna J Med Biotechnol 5(3):193–201
Huang L, Ruan H, Gu W, Xu Z, Cen P, Fan L (2007) Functional expression and purification of bovine enterokinase light chain in recombinant Escherichia coli. Prep Biochem Biotechnol 37(3):205–217. https://doi.org/10.1080/10826060701386695
Jouishomme H, Whitfield JF, Gagnon L, Maclean S, Isaacs R, Chakravarthy B, Durkin J, Neugebauer W, Willick G, Rixon RH (1994) Further definition of the protein kinase C activation domain of the parathyroid hormone. J Bone Miner Res 9:943–949. https://doi.org/10.1002/jbmr.5650090620
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8(8):1668–1674. https://doi.org/10.1110/ps.8.8.1668
Kraenzlin ME, Meier C (2011) Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol 7(11):647. https://doi.org/10.1038/nrendo.2011.108
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680
Malik A, Alsenaidy AM, Elrobh M, Khan W, Alanazi MS, Bazzi MD (2016) Optimization of expression and purification of HSPA6 protein from Camelusdromedarius in E. coli. Saudi J Biol Sci 23(3):410–419. https://doi.org/10.1016/j.sjbs.2015.04.017
Mongre RK, Singh Sodhi SS, Ghosh M, Kim JH, Kim N, Sharma N, Jeong DK (2014) A new paradigm to mitigate osteosarcoma by regulation of microRNAs and suppression of the NF-κB signaling cascade. Dev Reprod 18(4):197–212
Morley P, Whitfield JF, Willick GE (2012) Anabolic effects of parathyroid hormone on bone. Trends Endocrinol Metab 8:225–231. https://doi.org/10.1016/S1043-2760(97)00060-X
Mueller F, Moussa M, El Ghazaly M, Rohde J, Bartsch N, Parthier A, Kensy F (2013) Efficient production of recombinant parathyroid hormone (rPTH) fragment 1-34 in the methylotrophic yeast Hansenula polymorpha. GaBI J 2:114–122. https://doi.org/10.5639/gabij.2013.0203.035
Murray TM, Rao LG, Divieti P, Bringhurst FR (2004) Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev 26(1):78–113. https://doi.org/10.1210/er.2003-0024
Niu LX, Li JY, Ji XX, Yang BS (2015) Efficient expression and purification of recombinant human enteropeptidase light chain in Escherichia coli. Braz Arch Biol Technol 58(2):154–165. https://doi.org/10.1590/S1516-8913201400094
Pulicherla KK, Kumar PS, ManideepK, Rekha VPB, Ghosh M, Rao KRSS (2013) Statistical approach for the enhanced production of cold-active β-galactosidase from Thalassospira frigidphilosprofundus: a novel marine psychrophile from deep waters of Bay of Bengal. Prep Biochem Biotechnol 43(8):766–780. https://doi.org/10.1080/10826068.2013.773341
Rekha VPB, Ghosh M, Adapa V, Oh SJ, Pulicherla KK, Rao KRSS (2013) Optimization of polygalacturonase production from a newly isolated Thalassospira frigidphilosprofundus to use in pectin hydrolysis: statistical approach. Biomed Res Int 2013:750187. https://doi.org/10.1155/2013/750187
Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP (2011) Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Investig 34(10):801–810. https://doi.org/10.3275/7925
Stratford R Jr, Vu C, Sakon J, Katikaneni R, Gensure R, Ponnapakkam T (2014) Pharmacokinetics in rats of a long-acting human parathyroid hormone–collagen binding domain peptide construct. J Pharm Sci 103(2):768–775. https://doi.org/10.1002/jps.23843
Sung WL, Chan BS, Luk CK, Zahab DM, Willick GE, Barbier JR, Isaacs R, Maclean S, Ross V, Morley P, Whitfield JF (2000) High-yield expression of fully bioactive N-terminal parathyroid hormone analog in Escherichia coli. IUBMB Life 49(2):131–135. https://doi.org/10.1080/15216540050022458
Swarthout JT, D’Alonzo RC, Selvamurugan N, Partridge NC (2002) Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene 282(1):1–17. https://doi.org/10.1016/S0378-1119(01)00798-3
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Wingender E, Bercz G, Blocker H, Frank R, Mayer H (1989) Expression of human parathyroid hormone in Escherichia coli. J Biol Chem 264(8):4367–4373
Yamashita H, Gao P, Cantor T, Futata T, Murakami T, Uchino S, Watanabe S, Kawamoto H, Fukagawa M, Noguchi S (2003) Large carboxy-terminal parathyroid hormone (PTH) fragment with a relatively longer half-life than 1-84 PTH is secreted directly from the parathyroid gland in humans. Eur J Endocrinol 149(4):301–306. https://doi.org/10.1530/eje.0.1490301
Yari S, Behzadian F, Nejad HR, Masoumian M, Karimi M (2017) Expression and purification of soluble form of human parathyroid hormone (rhPTH1-34) by trx tag in E. coli. Res Mol Med 5(3):26–31. https://doi.org/10.29252/rmm.5.3.26
Acknowledgements
Authors thank Acharya Nagarjuna University, Andhra Pradesh for providing all facilities required for this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rajeenkanna Chilakapati and Chanchal Thomas Mannully carried out this experiment. Mrinmoy Ghosh arrange the data and stastical presentation. K.K. Pulicherla planned the experiment. There was no conflict of interest among the authors.
Research Involving Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10989_2019_9819_MOESM1_ESM.tif
Supplementary Figure S1 (a) Amplification of PTH gene of interest by PCR, (b) Screening (Colony PCR) of GST-PTH in pGEX-4T-3 recombinant gene containing transformants, (c) Enterokinase gene, (d) Screening (Colony PCR) of Enterokinase in pMAL-c5E recombinant gene containing transformants (TIF 2263 KB)
10989_2019_9819_MOESM2_ESM.tif
Supplementary Figure S2 DNA sequencing result of recombinant clone of truncated PTH. Sequencing done for recombinant clones along with the flanking regions of vector using universal primers (T7) confirmed the presence of truncated PTH with Enterokinase cleavage site in pGEX-4T-3 vector (TIF 1165 KB)
Rights and permissions
About this article
Cite this article
Chilakapati, R., Mannully, C.T., Ghosh, M. et al. Characterization and Expression Profiling of Recombinant Parathyroid Hormone (rhPTH) Analog 1–34 in Escherichia coli, Precise with Enhanced Biological Activity. Int J Pept Res Ther 26, 93–105 (2020). https://doi.org/10.1007/s10989-019-09819-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09819-1