Abstract
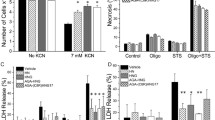
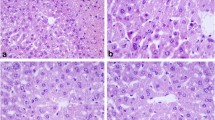
Until recently, necrotic cells death was considered an uncontrolled process. However, evidence was recently presented that necrosis is a regulated process associated with many clinical conditions. Humanin and its derivatives are peptides known for their anti-apoptotic activity against Alzheimer’s disease. Recently, the humanin-derivative AGA(C8R)-HNG17 (PAGASRLLLLTGEIDLP) was found to have protective effect against necrosis in traumatic brain injury model in mice. We have demonstrated now the protective effect of AGA(C8R)-HNG17 against necrosis in a dose dependent manner in HepG2 cells in vitro, where necrosis was induced in a glucose-free medium by chemohypoxia. Moreover, it was further demonstrated in a model of acetaminophen-induced liver injury in C57BL/6J male mice, in vivo. Intraperitoneal administration of the peptide at 10 and 30 mg/kg significantly prevented the increase in two plasma markers for necrosis, alanine aminotransferase (ALT, EC 2.6.1.2) and aspartate aminotransferase (AST, EC 2.6.1.1). Mitochondrial dysfunction is known to be the main cause of hepatic failure. Hence, the protection from liver injury by AGA(C8R)-HNG17, which we have recently found to target the mitochondria, may be mediated by mitochondrial regulation. Currently, there is no effective treatment for liver diseases, in which necrosis is involved. These findings may provide a new anti-necrotic strategy against APAP-induced liver injury and other liver diseases associated with necrosis using AGA(C8R)-HNG17 as a therapeutic agent.




Similar content being viewed by others
References
Almeda-Valdes P, Altamirano-Barrera A, Uribe M, Mendez-Sanchez N (2016) Metabolic features of alcoholic liver disease. Rev Recent Clin Trials 11:220–226
Chandok N, Watt K (2010) Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 85:451–458
Cohen A, Lerner-Yardeni J, Meridor D, Parola AH, Kasher R et al (2015) Humanin-derivatives inhibit necrotic cell death in neurons. Mol Med 21:505–514
Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ et al (2011) N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab 31:1768–1777
Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ et al (2005) Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–887
Du K, Williams CD, McGill MR, Xie Y, Farhood A et al (2013) The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol 273:484–491
Feldman Z, Gurevitch B, Artru AA, Oppenheim A, Shohami E et al (1996) Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg 85:131–137
Giboney PT (2005) Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Phys 71:1105–1110
Graham GG, Scott KF, Day RO (2005) Tolerability of paracetamol. Drug Saf 28:227–240
Guicciardi ME, Malhi H, Mott JL, Gores GJ (2013) Apoptosis and necrosis in the liver. Compr Physiol 3:977–1010
Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–328
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S et al (2003) Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423:456–461
Harada M, Habata Y, Hosoya M, Nishi K, Fuji R et al (2004) N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun 324:255–261
Hashimoto Y, Ito Y, Niikura T, Shao Z, Hata M et al (2001a) Mechanisms of neuroprotection by a novel rescue factor Humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun 283:460–468
Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M et al (2001b) Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci 21:9235–9245
Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H et al (2001c) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98:6336–6341
Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M (2009) Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp-130. Mol Biol Cell 20:2864–2873
Howell BA, Siler SQ, Shoda LKM, Yang Y, Woodhead JL, Watkins PB (2014) A mechanistic model of drug-induced liver injury aids the interpretation of elevated liver transaminase levels in a phase I clinical trial. CPT 3:1–8
Jaeschke H, McGill MR, Ramachandran A (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44:88–106
Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A (2013) Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol 55:279–289
Jin H, Liu T, Wang W-X, Xu J-H, Yang P-B et al (2010) Protective effects of [Gly14]-humanin on β-amyloid-induced PC12 cell death by preventing mitochondrial dysfunction. Neurochem Int 56:417–423
Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187:195–202
Korzeniewski C, Callewaert DM (1983) An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320
Latchoumycandane C, Goh CW, Ong MMK, Boelsterli UA (2007) Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology (Hoboken) 45:412–421
Lavanchy D (2009) The global burden of hepatitis C. Liver Int 29:74–81
Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H (1999) Inhibition of fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol 156:179–186
Lee C, Yen K, Cohen P (2013) Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab 24:222–228
Li L, Prabhakaran K, Mills EM, Borowitz JL, Isom GE (2005) Enhancement of cyanide-induced mitochondrial dysfunction and cortical cell necrosis by uncoupling protein-2. Toxicol Sci 86:116–124
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver:a tale of two deaths? Hepatology 43:S31-44
Masubuchi Y, Suda C, Horie T (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–116
Matsuoka M, Hashimoto Y (2010) Humanin and the receptors for humanin. Mol Neurobiol 41:22–28
Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ et al (2009) Humanin: a novel central regulator of peripheral insulin action. PLoS ONE 4:e6334
Niikura T, Chiba T, Aiso S, Matsuoka M, Nishimoto I (2004) Humanin: after the discovery. Mol Neurobiol 30:327–340
Parola AH, Nathan I, Kasher R, Lerner-Yardeni J, Cohen A (2011) Methods for inhibiting necrosis using humanin derivatives. PCT international applications WO2011104708
Prabhakaran K, Li L, Borowitz JL, Isom GE (2002) Cyanide induces different modes of death in cortical and mesencephalon cells. J Pharmacol Exp Ther 303:510–519
Rosser BG, Gores GJ (1995) Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology 108:252–275
Sandilands EA, Bateman DN (2009) Adverse reactions associated with acetylcysteine. Clin Toxicol 47:81–88
Tomishima Y, Ishitsuka Y, Matsunaga N, Nagatome M, Furusho H et al (2013) Ozagrel hydrochloride, a selective thromboxane A2 synthase inhibitor, alleviates liver injury induced by acetaminophen overdose in mice. BMC Gastroenterol 13:21
Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H (2014) Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol 275:122–133
Yang R, Zou X, Koskinen M-L, Tenhunen J (2012) Ethyl pyruvate reduces liver injury at early phase but impairs regeneration at late phase in acetaminophen overdose. Crit Care 16:R9
Yin H, Cheng L, Holt M, Hail N Jr, MacLaren R, Ju C (2010) Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology (Hoboken) 51:1007–1016
Ying G, Iribarren P, Zhou Y, Gong W, Zhang N et al (2004) Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol 172:7078–7085
Zelig U, Kapelushnik J, Moreh R, Mordechai S, Nathan I (2009) Diagnosis of cell death by means of infrared spectroscopy. Biophys J 97:2107–21014
Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC et al (2005) Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem 280:15815–15824
Zhang L, Gavin T, Geohagen BC, Liu Q, Downey KJ, LoPachin RM (2013) Protective properties of 2-acetylcyclopentanone in a mouse model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther 346:259–269
Zhou H, Chen L, Gao X, Luo B, Chen J (2012) Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J Neuropathol Exp Neurol 71:348–359
Acknowledgements
The authors thank Dr. Mark M Karpasas and Lina Saveliev from the Analytical Research Services Unit, Ben Gurion University of the Negev, for mass spectrometric analysis of the peptide, and Dr. Nurit Hadad for her help in the in vivo experiments. The financial support of the Kamin Program from the Chief Scientist of the Ministry of Economy of Israel (to AH Parola, I Nathan, and R Kasher), the James-Frank Center for Laser-Matter Interaction (to AH Parola), the Edmund Safra Foundation for Functional Bio-polymer, the New-York University Shanghai (NYUSH) research grant (to AH Parola), the Lyonel Israels’ Chair Fund (I Nathan), and the Pratt postdoctoral fellowships (to Meridor D. and Khalfin B.) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal procedures and care techniques were approved by the Ben-Gurion University of the Negev Committee for the Ethical Care and Use of Animals in Research.
Research Involving Human and Animal Participants
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Meridor, D., Cohen, A., Khalfin, B. et al. The Protective Effect of Humanin Derivative AGA(C8R)-HNG17 Against Acetaminophen-Induced Liver Injury in Mice. Int J Pept Res Ther 25, 565–571 (2019). https://doi.org/10.1007/s10989-018-9700-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9700-2