Abstract
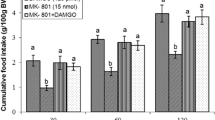
The present study was designed to examine the interconnection between oxytocinergic and glutamatergic systems on feeding behavior in neonatal chicks. In experiment 1, FD3 chicken ICV injected with (A) saline (control solution), (B) oxytocin (10 µg), (C) MK-801(NMDA receptor antagonist, 15 nmol) and (D) oxytocin + MK-801. In experiment 2, (A) control solution, (B) oxytocin (10 µg), (C) CNQX (390 nmol) and (D) oxytocin + CNQX were injected. Experiments 3–6 were similar to experiment 1, except FD3 chicks ICV injected with CNQX (AMPA glutamate receptors antagonist, 390 nmol), AIDA (mGLUR1 glutamate receptors antagonist, 2 nmol), LY341495 (mGLUR2 glutamate receptors antagonist, 150 nmol) and UBP1112 (mGLUR3 glutamate receptors antagonist, 2 nmol) instead of MK-801, respectively. In experiment 7, injections were (A) control solution, (B) glutamate (300 nmol), (C) ABN-297 (9 nmol) and (D) glutamate + ABN-297. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of glutamate decreased food intake and co-injection of the glutamate + ABN-297 increased food consumption in neonatal broiler (P < 0.001). ICV injection of oxytocin, significantly decreased food intake in neonatal chicks (P < 0.001). Also, MK-801 significantly attenuated oxytocin- induced hypophagia in chicks (P < 0.001). In addition, combination of oxytocin plus CNQX significantly decreased hypophagic effect of the oxytocin in neonatal chicks (P < 0.001). These results suggested interconnection between central oxytocinergic and glutamatergic systems on feeding behavior is probably mediated via NMDA and AMPA receptors.








Similar content being viewed by others
References
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun 39:151–157
Amano T, Unal CT, Pare D (2010) Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13:489–494
Arletti R, Benelli A, Bertolini A (1989) Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93
Babygirija R, Zheng J, Ludwig K, Takahashi T (2010) Central oxytocin is involved in restoring impaired gastric motility following repeated stress in mice. Am J Physiol Regul Integr Comp Physiol 298:R157-R165
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res 62(4):125–129
Baracz SJ, Everett NA, McGregorI S, Cornish JL (2014) Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behavior in rats. AddictBiol. https://doi.org/10.1111/adb.12198
Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71:277–282
Charles JR, Duva MA, Ramirez GJ, Lara RL, Yang CR, Stanley BG (2014) Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacol 79:59–65
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121
Da Silva AA, Marino-Neto J, Paschoalini MA (2003) Feeding induced by microinjections of NMDA and AMPA–kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain res 966:76–83
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Hettes SR, Gonzaga WJ, Heyming TW, Nguyen JK, Perez S, Stanley BG (2010) Stimulation of lateral hypothalamic AMPA receptors may induce feeding in rats. Brain Res 1346:112–120
Jo YH, Stoeckel ME, Freund-Mercier MJ, Schlichter R (1998) Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci 18(7):2377–2386
Kania BF, Wrońska D (2015) Possible role of glutamate/oxytocin/GABA interactions during motivational stress in sheep. Austin Ther 2(2):1019
Klockars A, Levine AS, Olszewski PK (2015) Central oxytocin and food intake: focus on macronutrient-driven reward. Front Endocrinol 6:65. https://doi.org/10.3389/fendo.2015.00065
Kuo J, Hariri OR, Micevych P (2009) An interaction of oxytocin receptors with metabotropic glutamate receptors in hypothalamic astrocytes. J Neuroendocrinol 21(12):1001–1006
Ladepeche L, Yang L, Bouchet D, Groc L (2013) Regulation of dopamine D1 receptor dynamics within the postsynaptic density of hippocampal glutamate synapses. PLoS ONE 8(9):e74512
Ludwig M (1998) Dendritic release of vasopressin and oxytocin. J Neuroendocrinol 10:881–895
McFadden KL, Cornier MA, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol 5(553):1–7
Moos FC, Rossi K, Richard P (1997) Activation of N-methyl-d-aspartate receptors regulates basal electrical activity of oxytocin and vasopressin neurons in lactating rats. Neuroscience 77(4):993–1002
Morsette DJ, Sidorowicz H, Sladek CD (2001) Role of metabotropic glutamate receptors in vasopressin and oxytocin release. Am J Physiol Regulatory Integrative Comp Physiol 281:R452–R458
Mortezaei SS, Zendehdel M, Babapour V, Hasani K (2013) The role of glutamatergic and GABAergic systems on serotonin- induced feeding behavior in chicken. Vet Res Commun 37:303–310
Ninan I (2011) oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J neurochem 119:324–331
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J poult Sci 5(4):301–308
Pampillo M, Díaz MC, Duvilanski BH, Rettori V, Seilicovich A, Lasaga M (2001) Differential effects of glutamate agonists and d-aspartate on oxytocin release from hypothalamus and posterior pituitary of male rats. Endocrine 15(3):309–315
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hearing Res 236:52–60
Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F et al (2012) Oxytocin Regulates changes of extracellular glutamate and GABA levels induced by Methamphetamine in the mouse brain. Addict Biol 17:758–769
Richards MP (2003) Genetic regulation of feed intake and energy balance in poultry. Poult Sci 82(6):907–916
Sabatier N, Leng G, Menzies J (2013) Oxytocin, feeding, and satiety. Front Endocrinol 4:35
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ (2015) Oxytocin reduces amygdala activity, increases social interactions and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav Neurosci 129(4):389–398
Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE (1999) Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci 19(9):3367–3375
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DLAP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Torkzaban M, Zendehdel M, Babapour V, Panahi N, Hassanpour S (2017) Interaction between central opioidergic and glutamatergic systems on food intake in neonatal chicks: role of NMDA, AMPA and mGLU1 receptors. Int J Pept Res Ther. https://doi.org/10.1007/s10989-017-9601-9
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA (2000) Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav 65(1):67–74
Acknowledgements
The authors thank the central laboratory (Dr. Rastegar Lab.) of the Faculty of Veterinary Medicine, University of Tehran for cooperation. This research is conducted as a part of the PhD thesis of the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Research Involving Human and Animal Rights
All experiments were executed according to the Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Jalali, M., Zendehdel, M., Babapour, V. et al. Interaction Between Central Oxytocinergic and Glutamatergic Systems on Food Intake in Neonatal Chicks: Role of NMDA and AMPA Receptors. Int J Pept Res Ther 25, 195–203 (2019). https://doi.org/10.1007/s10989-017-9664-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9664-7