Abstract
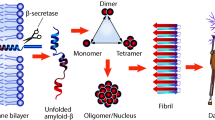
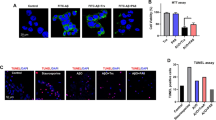
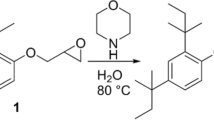
Aggregation of Amyloid β (Aβ) in the interneuronal spaces is a major etiopathological factor for onset and progression of Alzheimer’s disease (AD). Since the mechanism of aggregation is not fully understood, control and modulation of the aggregation process is a challenging task. Although, several strategies were developed for the past few decades, yet there is no proper therapeutics available. Herein, we report a peptide based pro-drug, termed as a conformational Pro-Drug peptide (PDp), which disrupts existing Aβ fibrils, but does not produce toxic soluble oligomers, through a series of spontaneous chemical reactions resulting in in situ generation of β-sheet destabilizing factors. Furthermore, PDp reduces Aβ mediated toxicity examined on an in vitro model consisting of the human neuroblastoma SH-SY5Y cells. PDp also disrupts fibrils originated from AD affected human cerebrospinal fluid. These findings will help to understand the process of amyloidogenesis better and also indicate a novel approach for therapeutically important peptide design.






Similar content being viewed by others
References
Agholme L, Lindstrom T, Kagedal K, Marcusson J, Hallbeck M (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis 20:1069–1082
Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K (1999) Cerebrospinal fluid β-Amyloid(1–42) in Alzheimer disease. Arch Neurol 56:673–680
Blennow K (2004) Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx 1:213–225
Chen S, Wetzel R (2001) Solubilization and disaggregation of polyglutamine peptides. Protein Sci 10:887–891
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Clarke S (1987) Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res 30:808–821
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Larson ME, Lesne SE (2012) Soluble Aβ oligomer production and toxicity. J Neurochem 120:125–139
Liu Q, Xie X, Emadi S, Sierks MR, Wu J (2015a) A novel nicotinic mechanism underlies β-amyloid-induced neurotoxicity. Neuropharmacology 97:457–463
Liu YH, Bu XL, Liang CR, Wang YR, Zhang T, Jiao SS, Zeng F, Yao XQ, Zhou HD, Deng J, Wang YJ (2015b) An N-terminal antibody promotes the transformation of amyloid fibrils into oligomers and enhances the neurotoxicity of amyloid-beta: the dust-raising effect. J Neuroinflammation 12:153
Mark RJ, Hensley K, Butterfield DA, Mattson MP (1995) Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. Neuroscience 15:6239–6249
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Muthuraj B, Layek S, Balaji SN, Trivedi V, Iyer PK (2015) Multiple function fluorescein probe performs metal chelation, disaggregation, and modulation of aggregated Aβ and Aβ-Cu complex. ACS Chem Neurosci 6:1880–1891
Nadimpally KC, Paul A, Mandal B (2014) Reversal of aggregation using β-breaker dipeptide containing peptides: Application to Aβ(1–40) self-assembly and its inhibition. ACS Chem Neurosci 5:400–408
Nicolas E, Pedroso E, Giralt E (1989) Formation of aspartimide peptides in Asp-Gly sequences. Tetrahedron Lett 30:497–500
Nunan J, Small DH (2000) Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett 483:6–10
Park YH, Kim YJ, Son H, Yang HD (2009) Inhibition of β-amyloid1-40 peptide aggregation and neurotoxicity by Citrate. Korean J Physiol Pharmacol 13:273–279
Paul A, Nadimpally KC, Mondal T, Thalluri K, Mandal B (2015) Inhibition of Alzheimer’s amyloid-β peptide aggregation and its disruption by a conformationally restricted α/β hybrid peptide. Chem Commun 51:2245–2248
Sclip A, Antoniou X, Colombo A, Camici GG, Pozzi L, Cardinetti D, Feligioni M, Veglianese P, Bahlmann FH, Cervo L (2011) c-Jun N-terminal kinase regulates soluble Aβ oligomers and cognitive impairment in AD mouse model. J Biol Chem 286:43871–43880
Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C et al (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359:325–327
Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castano EM, Frangione B (1998) Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy. Nature Med 4:822–826
Squires AM, Devlin GL, Gras SL, Tickler AK, MacPhee CE, Dobson CM (2006) X-ray scattering study of the effect of hydration on the cross-β structure of Amyloid Fibrils. J Am Chem Soc 128:11738–11739
Tjernberg LO, Näslund J, Lindqvist F, Johansson J, Karlström AR, Thyberg J, Terenius L, Nordstedt C (1996) Arrest of-Amyloid Fibril Formation by a Pentapeptide Ligand. J Biol Chem 271:8545–8548
Toniolo C, Crisma M, Moretto A, Peggion C, Formaggio F, Aleman C, Cativiela C, Ramakrishnan C, Balaram P (2015) Peptide δ-Turn: literature survey and recent progress. Chem Eur J 21:13866–13877
Wang H, Xu Y, Yan J, Zhao X, Sun X, Zhang Y, Guo J, Zhu C (2009) Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res 1283:139–147
Williams TL, Day IJ, Serpell LC (2010) The effect of Alzheimer’s Aβ aggregation state on the permeation of biomimetic lipid vesicles. Langmuir 26:17260–17268
Zheng L, Minguez AC, Hallbeck M, Jerhammar F, Marcusson J, Terman A (2012) Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system. Transl Neurodegener 1:19
Zheng X, Xie Z, Zhu Z, Liu Z, Wang Y, Wei L, Yang H, Yang H, Liu Y, Bi J (2014) Methyllycaconitine Alleviates Amyloid-β Peptides-Induced Cytotoxicity in SH-SY5Y Cells. PLoS ONE 9:e111536
Acknowledgements
This work was financially supported by the grants from DBT (NER-BPMC) [BT/347/NE/TBP/2012 & BT/PR16164/NER/95/88/2015] to BM & ACM and DST PURSE-II, UPE-II (Project Id No. 247), UGC RN, UGC DRS-I to ACM, Govt. of India. We thank CIF, IIT Guwahati for LC-MS, TEM and FESEM studies, and School of Life Sciences, Jawaharlal Nehru University, New Delhi for their kind co-operations.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, A., Kumar, S., Kalita, S. et al. A Peptide Based Pro-drug Disrupts Alzheimer’s Amyloid into Non-toxic Species and Reduces Aβ Induced Toxicity In Vitro. Int J Pept Res Ther 24, 201–211 (2018). https://doi.org/10.1007/s10989-017-9602-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9602-8